
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our customタンパク質 services!
カスタムサービスについてのお問い合わせはこちら >>



























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Insights > Human Erythropoietin and its use in LNP-mediated mRNA Drug Discovery Erythropoietin (EPO) is a 30.4 kDa glycoprotein composed of a single 165 amino acid residues chain to which has four glycosylation sites (Figure 1). This hormone is naturally produced from the kidneys to stimulate the production of red blood cells.
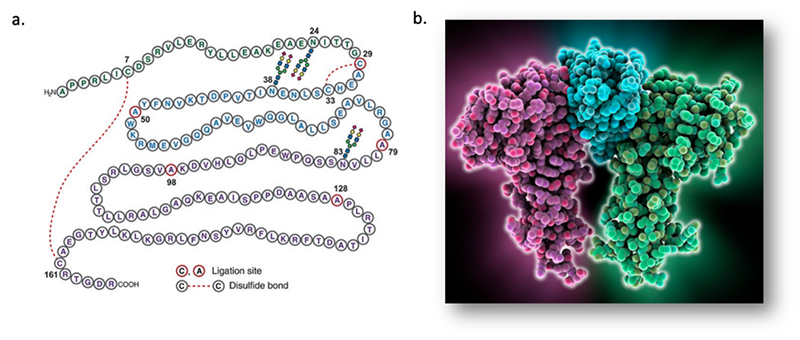
Figure 1. The structure of human EPO. a, the primary structure of EPO (ref 1 )(https://doi: 10.1126/sciadv.1500678); b, the 3D structure of EPO.
LNP-mediated mRNA drugs refer to a class of therapeutic agents that utilize lipid nanoparticle (LNP) technology to deliver modified messenger RNA (mRNA) into cells. This innovative approach has gained considerable attention in the field of biopharmaceutics for its versatility in gene delivery, including:
1. Vaccines: LNP-mediated mRNA technology has been notably successful in the development of mRNA vaccines, such as the Pfizer-BioNTech and Moderna COVID-19 vaccines. These vaccines use synthetic mRNA to instruct cells to produce a viral spike protein, eliciting an immune response and gifting long-term protection against the virus.
2. Gene Therapy: LNP-mediated mRNA enables the delivery of therapeutic gene-editing molecules to correct genetic defects or promote the production of specific proteins.
3. Protein Replacement Therapy: LNP-mediated mRNA drugs can be designed to instruct cells to produce missing or deficient proteins, offering a potential treatment for certain genetic disorders with misfolded protein production or non-expressing genes.
LNPs serve as the mRNA’s carriers in LNP-mediated drug development, protecting it from degradation and facilitating its entry into target cells. The lipid coating allows the LNPs to merge with the cell membrane, releasing the mRNA into the cytoplasm. When evaluating LNP-mediated mRNA delivery efficacy and putatively pharmacologic effects of drugs in vivo / in vitro, the use of human EPO (hEPO) -encoding mRNA is required (Figure 2).
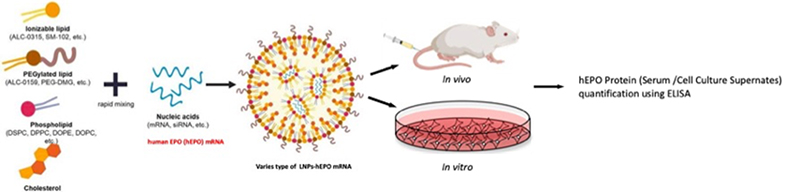
Figure 2. The mRNA delivery efficacy and pharmacology evaluation of mRNA-LNPs by modified mRNA encoding the protein for human erythropoietin.
As such, this makes EPO the ideal molecule to quantify mRNA delivery from LNPs. For example, researchers used LNPs (iLP171) loaded with various amounts of modified hEPO-mRNA to transfect Huh7 cells and investigate the protein expression of mRNA-LNPs in vitro. EPO secreted from the cells is then quantified by ELISA was used to assess hEPO expression at specific time points. These findings showed that hEPO could effectively express by Huh7 cells in a dose-dependent and time-dependent manner (Figure 3).
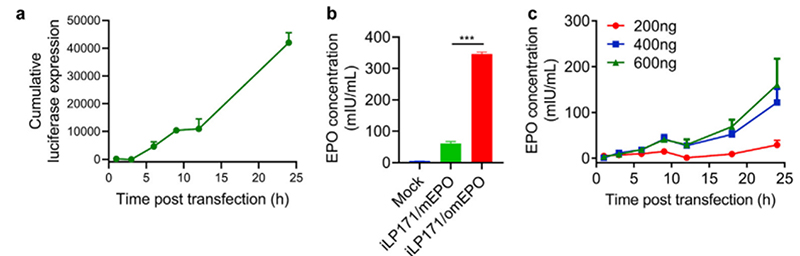
Figure 3. In vitro protein expression mediated by iLP171/mRNA. (ref 2 https://doi.org/10.1016/j.bioactmat.2020.07.003)
In a separate study, researchers also sought to evaluate the efficacy of various lipid nanoparticles (LNPs) in delivering mRNA encoding a therapeutic protein in vivo. Specifically, mice were administered with different types of LNPs carrying modified hEPO-mRNA. Following a defined timeframe, the concentration of hEPO in mouse serum was quantified using the ELISA method (Figure 4). hEPO production following i.v. administration of 20% DODAP liver SORT LNPs peaked at 6 h and was maintained for more than 1 week (ref 3).
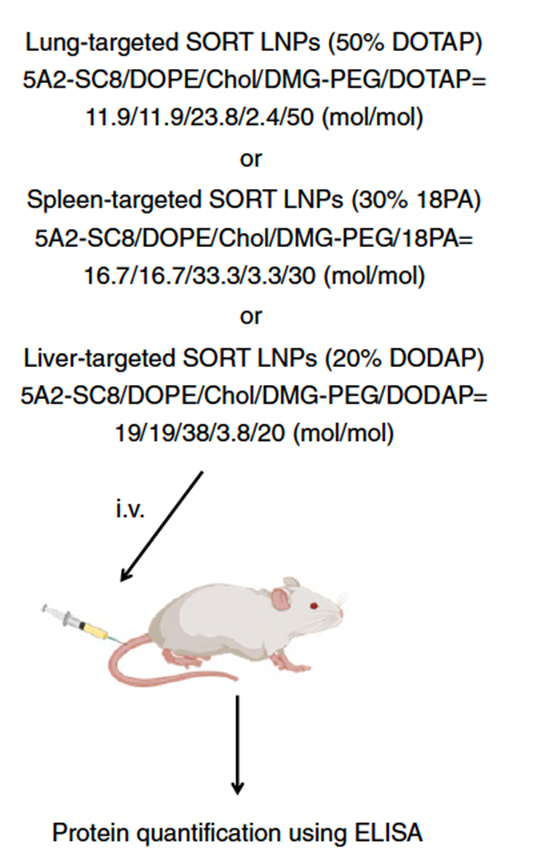
Figure 4. Scheme for mRNA delivery of secreted proteins in vivo. (ref 3 https://doi.org/10.1038/s41565-020-0669-6)
This approach not only enabled the evaluation of mRNA transcription and protein expression based on LNPs in vivo but also extended to in vitro settings, where ELISA assays were utilized to measure hEPO levels in cell supernatant, serum, and plasma.
Due to its consistency and stability both in vivo and in vitro, Human EPO is a valuable secreted protein for assessing both mRNA delivery and putatively pharmacologic effects of LNP-mediated mRNA drugs.
Additionally, in rat and monkey species, modified mRNA encoding hEPO had predictable and consistent pharmacologic and toxicologic effects. Pharmacokinetic analysis conducted following the first dose showed that measured hEPO levels were maximal at 6 hours after the end of intravenous infusion and in excess of 100-fold the anticipated efficacious exposure (17.6 ng/ml) at the highest dose tested (ref 4 journals.sagepub.com/doi/10.1177/0300985817738095). Overall, these studies indicate that EPO serves as a crucial marker for evaluating the mRNA delivery efficacy and pharmacokinetics profile of when utilizing LNP-based delivery of therapeutic agents.
This web search service is supported by Google Inc.






