 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Interferon (IFN) Family Proteins

Interferon (IFN) was first discovered in 1957 by Alick Isaacs and Jean Lindenmann from their initial studies in viral interference. Over the years, various studies about IFN were performed, elucidating its anti-viral activity. It was only until 1980 when IFN could be produced in a large-scale for research use, where pioneering recombinant DNA technology was utilized. IFN proteins were discovered to have both anti-tumor and immunomodulatory functions along with its inherent anti-viral activity, classifying them as a subset of cytokines.
The IFN protein family is organized into three types according to its corresponding receptor: type I, type II and type III; each of which are produced in different cellular locations. IFN-α and IFN-β are the most prominent members of type I IFNs and are mainly produced by pathogenesis-associated molecular patterns (PAMPs). PAMPs are induced by stimulation of Toll-like receptors (TLR) or cytoplasmic pattern recognition receptors located on the cell membrane. Type II IFNs have only one type, IFN-γ, which are produced by a variety of cells in the immune system. This includes innate lymphoid-like cell populations such as innate lymphocytes (ILC) and natural killer (NK) cells, as well as adaptive immune cells consisting of helper T cells 1 (Th1) and CD8 cytotoxic T lymphocytes (CTL). Type III IFNs are mainly produced by epithelial cells in non-hematopoietic cells, where viruses can mediate type III interferon expression in different cell types. However, since these IFNs were discovered in 2003, the exact mechanism of its production is still unknown and is the subject of various academic studies.
The corresponding signaling pathways of the three types of IFNs are also different, where each bind to different compositions of heterodimeric receptor complexes. Intracellular signaling is conducted through the Janus kinase/signal transducer and acts as an activator of transcription (JAK/STAT) pathways.
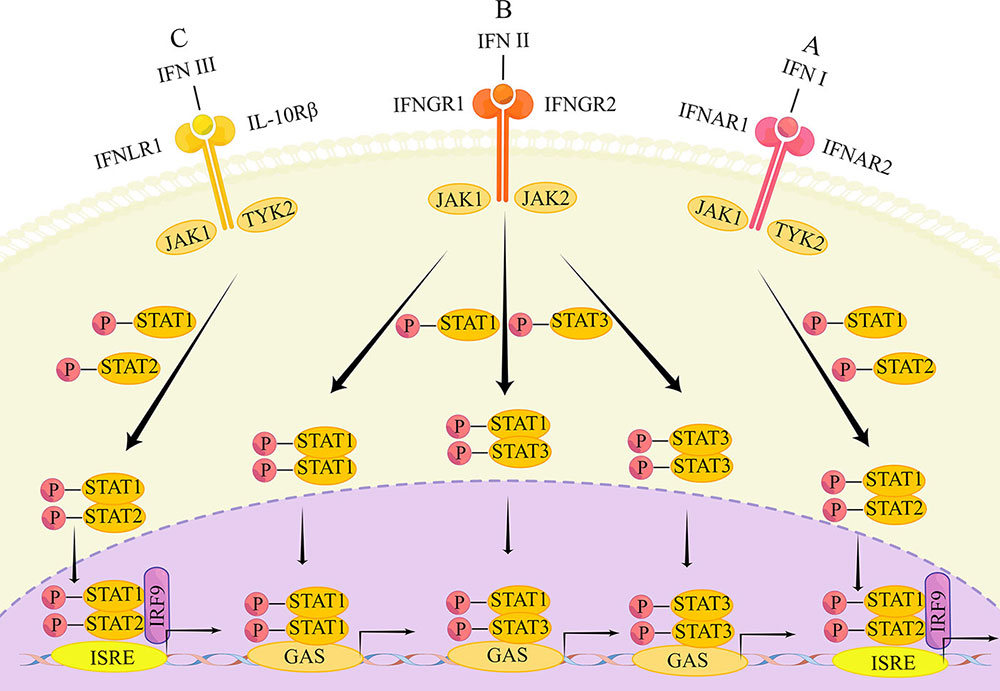
The main transduction pathways of the IFN signaling
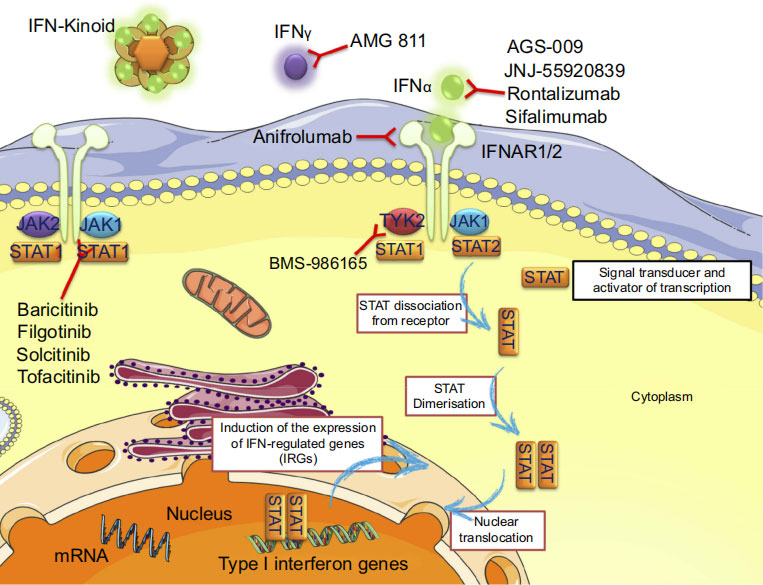
Mechanism of action of a drug targeting IFN (Anifrolumab)
Due to the widespread effects of the IFN regulatory pathways, IFN is often associated with the progression of tumor diseases. In vitro studies have shown that IFN can inhibit tumor cell growth by up-regulating the cell cycle and also induce apoptosis by binding to tumor necrosis factor-related apoptosis-inducing ligands. However, in vivo studies have shown that deletion of the type I or type II interferon signaling pathway accelerates tumorigenesis and progression.
The correlation of both type I and II IFN responses to oncogenic properties has highlighted it as a significant pathway for targeted cancer drug resistance. This was observed from the resulting tumor cells after cancer therapy expressing intact or partially intact IFN signaling. As a result, a resistance to viral replication is developed, preventing targeted therapeutics from exerting its normal anti-tumor effects. To combat this, targeted therapies inhibiting JAK/STAT signaling by IFN can be a therapeutic modality to overcome resistance to therapeutic agents.
To assist with your research into the IFN protein family, ACROBiosystems provides a comprehensive catalog of high-quality IFNs to meet your needs in drug discovery, functional evaluation, quality control.
![]() HEK293 expressed, native protein conformation
HEK293 expressed, native protein conformation
![]() High purity verified by SDS-PAGE
High purity verified by SDS-PAGE
![]() High structural homogeneity verified by SEC-MALS
High structural homogeneity verified by SEC-MALS
![]() High bioactivity verified by ELISA/SPR/BLI - protocol available
High bioactivity verified by ELISA/SPR/BLI - protocol available
![]() High batch-to-batch consistency
High batch-to-batch consistency
| Molecule | Cat. No. | Species | Product Description | Preorder/Order |
|---|
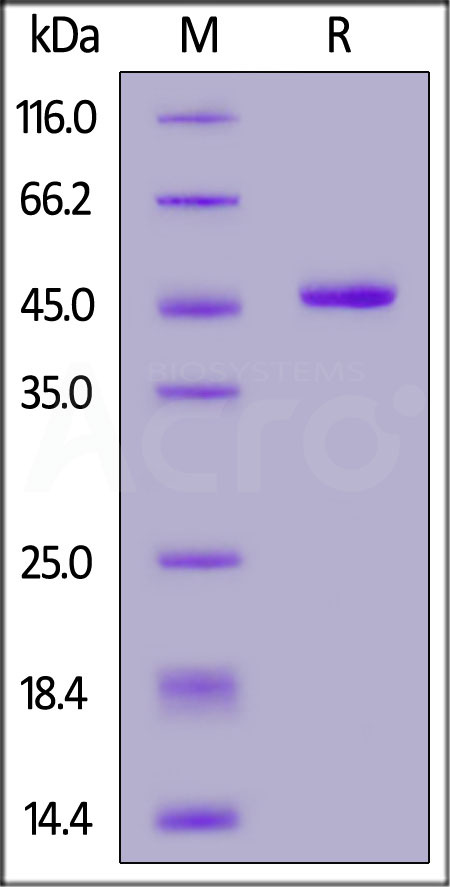
Human IFN-alpha 1 (Cat. No. IFA-H5258), Fc Tag on SDS-PAGE under reducing (R) condition. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%.
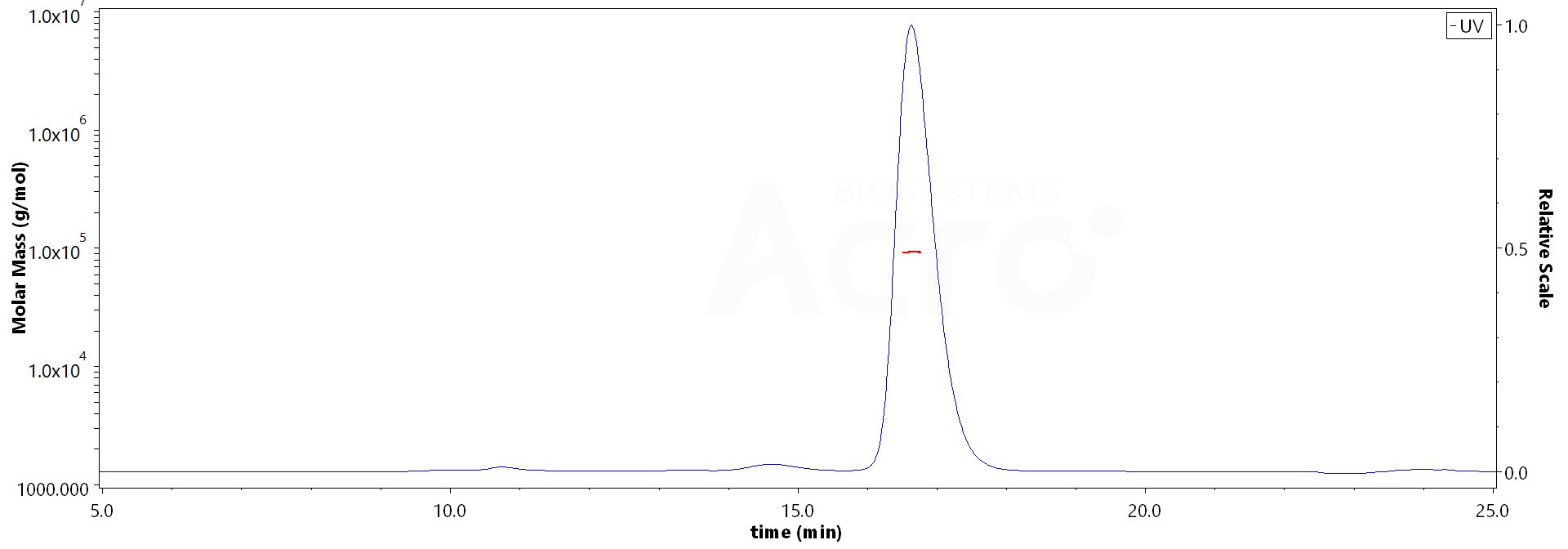
The purity of Human IFN-alpha 1, Fc Tag (Cat. No. IFA-H5258) is more than 90% and the molecular weight of this protein is around 90-118 kDa verified by SEC-MALS.
This web search service is supported by Google Inc.