 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> resDetecTM CGT CMC Manufacturing Process Residue Detection Solutions

Browse all our resDetect™ products
Cell and Gene therapies (CGT) face significant manufacturing challenges due to their complex processes, intrinsic safety risks, and regulatory compliance mandates. Throughout the CMC (Chemistry, Manufacturing, and Controls) production phase, residual materials such as ancillary/raw materials, additives, and host cell DNA can persist as impurities, posing safety and efficacy concerns for the final products. Stringent regulations necessitate comprehensive testing and clearance of these process-related residuals to ensure both quality and regulatory compliance. Robust residuals testing strategies are therefore essential for the successful development of CGT therapies.
Our brand, resDetect™, offers comprehensive solutions for quality control of process-related residuals, empowering and supporting your CGT drug discovery journey.
 Stable and Reliable — Stringent batch testing and release, with inter-batch differences less than 15%.
Stable and Reliable — Stringent batch testing and release, with inter-batch differences less than 15%. Regulatory Compliance — Complaint with standards and regulations of international regulatory authorities.
Regulatory Compliance — Complaint with standards and regulations of international regulatory authorities. Global Supply— Product delivery in 1-3 days, worldwide.
Global Supply— Product delivery in 1-3 days, worldwide. Thorough Validation — Referencing guidelines such as ICH Q2 (R2) and ChP 9101.
Thorough Validation — Referencing guidelines such as ICH Q2 (R2) and ChP 9101.Cell
Therapies
Gene
Therapies

E. coli resDNA Quantitative Kit (qPCR)
HEK293 resDNA Quantitation Kit (qPCR)
High sensitivity, high consistency, and high specificity are achieved in the detection of residual host cell DNA in biopharmaceuticals, with a Limit of Lower Detection (LLOD) as low as 1 fg/µL.
High sensitivity, high consistency, and high specificity are achieved in the detection of residual plasmid DNA in biopharmaceuticals.
Accurately quantify the potentially hazardous specific transfection sequences (E1A and SV40 LTA) that may remain in biopharmaceuticals for safety assessment.
Accurately quantify the residual GMP raw material enzymes such as universal nucleases and Cas gene editing enzymes.
Perform detection for the residual DNA/RNA nucleases in the laboratory environment, materials, and products.
Human Interleukin-15 (IL-15) ELISA Kit
Human Interleukin-7 (IL-7) ELISA Kit
Human Interleukin-21 (IL-21) ELISA Kit
GMP-compliant residual quantification assay kits for cell activation and cultivation factors or antibodies, ensuring product safety for worry-free cultivation!
Competitive ELISA designed to achieve high sensitivity in detecting residual antibiotics in samples.

E. coli resDNA Quantitative Kit (qPCR)
HEK293 resDNA Quantitation Kit (qPCR)
High sensitivity, high consistency, and high specificity are achieved in the detection of residual host cell DNA in biopharmaceuticals, with a Limit of Lower Detection (LLOD) as low as 1 fg/µL.
High sensitivity, high consistency, and high specificity are achieved in the detection of residual plasmid DNA in biopharmaceuticals.
Accurately quantify the potentially hazardous specific transfection sequences (E1A and SV40 LTA) that may remain in biopharmaceuticals for safety assessment.
Accurately quantify the residual GMP raw material enzymes such as universal nucleases and Cas gene editing enzymes.
Perform detection for the residual DNA/RNA nucleases in the laboratory environment, materials, and products.
Utilizing a competitive ELISA method to achieve high sensitivity in detecting residual antibiotics in samples.
*If you have any other development needs for residue detection products, please feel free to contact us.
Enzyme Residue
Detection
Host Cell Residual
Nucleic Acid Detection
Plasmid DNA Residue
Detection
Viral Oncogenes
Residue Detection
Cytokine & Antibody
Residue Detection
Antibiotic Residue
Detection
In the CGT manufacturing, rigorous monitoring and analysis of residual nucleases and CRISPR/Cas proteins are imperative for ensuring product quality and safety. These biomolecules serve as critical quality indicators for CGT products. Additionally, residual DNases and RNases can trigger potent immunogenic responses and degrade therapeutic nucleic acids when present as impurities, compromising efficacy and safety.
ACROBiosystems' resDetect™ kits provides a comprehensive solution for reliable residual enzyme detection, a vital component of your CMC quality control process. Our high-performance kits enable sensitive and specific quantification of residual nucleases, CRISPR/Cas proteins, and other enzymes across CGT production workflows.
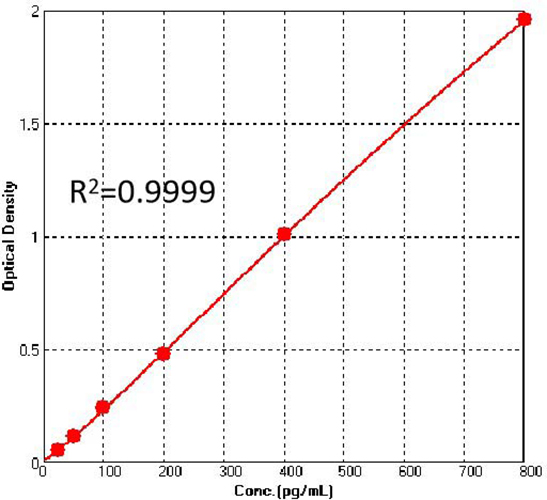
Figure 1. For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only. (Cat. No. CRS-A031)
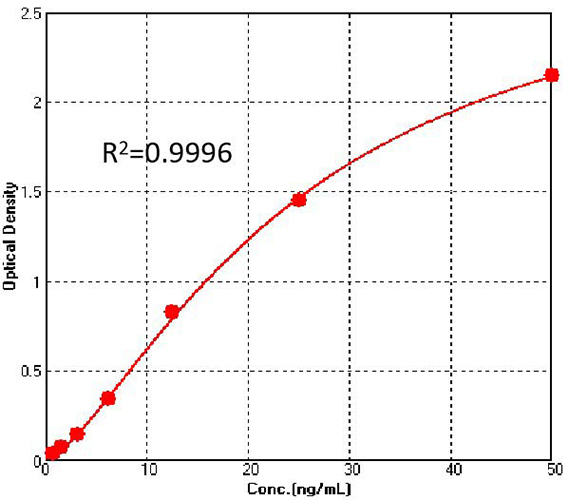
Figure 2. For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only. (Cat. No. CAS-C001)
 Products
Products resDetect™ GENIUS™ Nuclease ELISA Kit
resDetect™ GENIUS™ Nuclease ELISA Kit
 resDetect™ Cas9 (CRISPR Associated Protein 9) ELISA Kit
resDetect™ Cas9 (CRISPR Associated Protein 9) ELISA Kit
 Resources
Resources Protocol——resDetect™ GENIUS™ Nuclease ELISA Kit
Protocol——resDetect™ GENIUS™ Nuclease ELISA Kit
 Brochure—resDetect™ CMC Manufacturing Process Residue Detection Solutions
Brochure—resDetect™ CMC Manufacturing Process Residue Detection Solutions
 FAQ
FAQThe excitation and emission wavelengths provided in the manual are intended as recommendations. These kits offer flexibility in terms of the wavelengths that can be used for fluorescence detection.
a) For the RNase Activity Assay kit: The fluorescence signals can be detected within the approximate range of 440nm to 510nm for excitation and 500nm to 570nm for emission. The fluorescence parameters are not restricted to Ex/Em = 490nm/520nm; any values within the specified ranges can be utilized without compromising the assay performance.
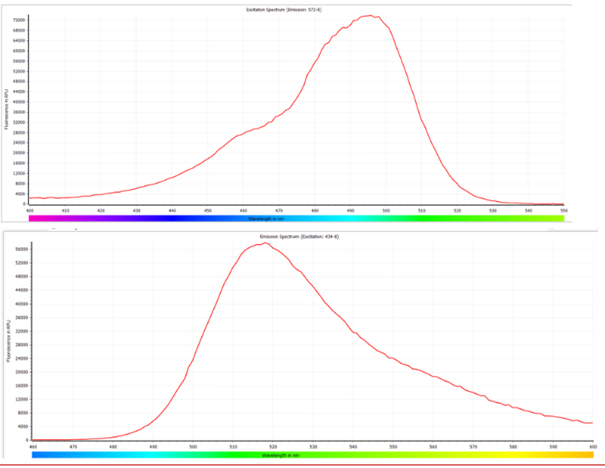
b) For DNase Activity Assay kit: The fluorescence signals can be detected within the approximate range of 520nm to 550nm for excitation and 540nm to 600nm for emission. Similarly, the fluorescence parameters are not limited to Ex/Em = 535nm/565nm; any values within the specified ranges can be employed for accurate detection.
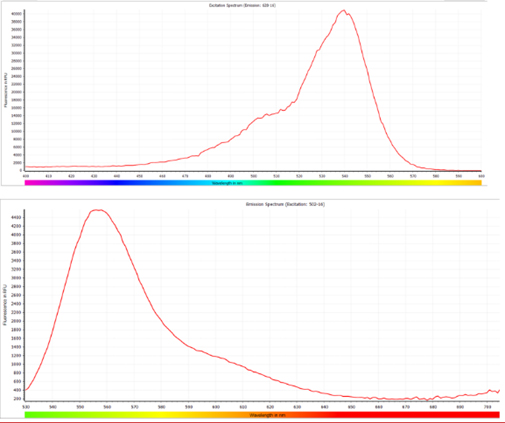
ACRO has conducted tests and recommends the following instruments for optimal performance. Additionally, most fluorescence-based instruments on the market that can accommodate the appropriate excitation and emission wavelength ranges should be compatible with our kits.
| Instrument Manufacturer | Instrument Model | Notes |
|---|---|---|
| BMG Labtech | Claristar Plus | Currently used by ACRO, capable of kinetic and endpoint reading, temperature control (e.g., incubation at 37°C) |
| BMG Labtech | omega | Mainframe with fluorescence capability |
| BMG Labtech | Vantastar | Mainframe with fluorescence capability |
| BMG Labtech | Vantastar F | Mainframe with fluorescence capability |
| Agilent | BioTek Synergy LX Multi-Mode Reader | Can only perform endpoint fluorescence measurement, not capable of kinetic measurement, and lacks temperature control capabilities |
The conversion factors for relating enzyme activity units to concentration are as follows:
a) For RNase activity: 1 pg of RNase is corresponds to 10⁻6 units (U) RNase activity.
b) For DNase activity: 2 ng of DNase corresponds to 1 unit (U) of DNase activity.
HEK293/HEK293T cells and E. coli are widely employed for viral vector and plasmid vector production in the large-scale manufacturing of CGTs. However, residual host cell DNA (resDNA) from these expression systems can persist through production, posing potential safety concerns, including potential carcinogenicity and infectious agents. To mitigate these risks and ensure safety, and efficacy of CGT therapies, host cell residual DNA detection is a critical part of quality control measurements during the CMC process.
resDetect™ offers a comprehensive range of highly sensitive residual host cell nucleic acid detection kits across various host cell types, providing robust support for your resDNA quality control needs.
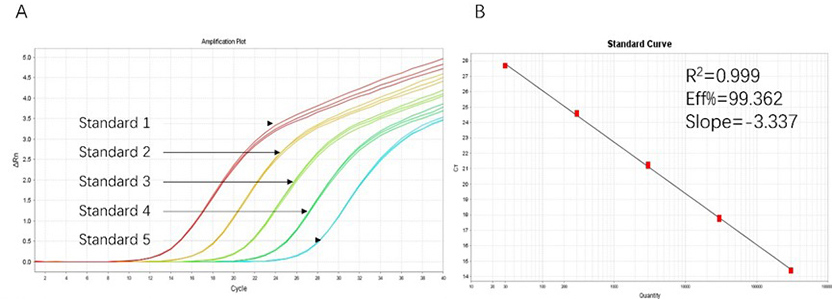
Figure 1. High sensitivity and broad dynamic range using the HEK293 resDNA Quantitation Kit. (A) Typical analysis results obtained with Standard 1 (300 pg/μL) to 5 (30 fg/μL). (B) The standard curve of the 10-fold dilution series. PCR efficiency should be 90-110%.
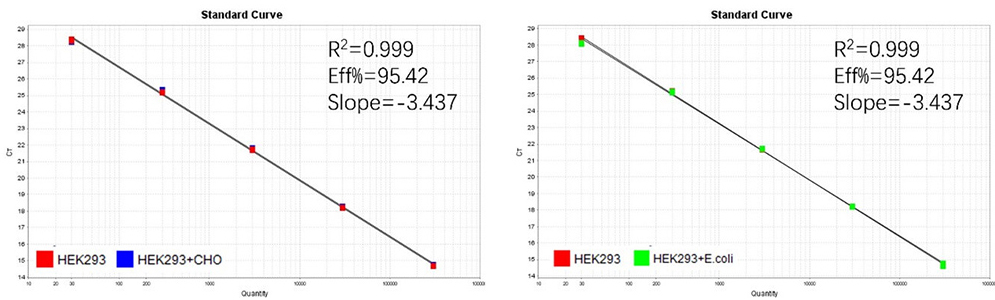
Figure 2. Assay specificity. Standard curves generated using 10-fold serial dilution of HEK293 genomic DNA (included in the kit) in the presence of CHO DNA and E. coli DNA.
 Products
Products resDetect™ E. coli resDNA Quantitative Kit (qPCR)
resDetect™ E. coli resDNA Quantitative Kit (qPCR)
 FAQ
FAQ1) The resDetect™ E. coli resDNA Quantitative kit achieves a Limit of Quantification (LOQ) of 3fg/μL through optimization, including:
• Optimization of trace nucleic acid extraction.
• Effective modification of primers and probes.
• Optimization of the system to achieve the best combination.
2) The resDetect™ E. coli standard material is internally produced by the company and traceable to reference materials from the US Pharmacopeia (USP).
1) The standard materials for HEK293 and HEK293T kits are sourced from ECACC (European Collection of Authenticated Cell Cultures).
2) The primary difference between these two kits lies in the standard material used; however, the primers and probes are identical. For applications involving HEK293T cells, employing the HEK293T genome as the standard for resDNA quantification is recommended for optimal accuracy.
3) To streamline the nucleic acid extraction process, we recommend using the resDetect™ resDNA Sample Preparation Kit Ⅱ (Magnetic Beads) (OPA-R024) with HEK293 or HEK293T kits."
One potential reason for qPCR amplification curves rising without exhibiting exponential growth could be due to the presence of residual ethanol carried over from the magnetic bead washing process. Even minor amounts of ethanol remaining with the beads can inhibit the PCR amplification reaction, leading to a suboptimal exponential amplification phase.
The amplicon size for these kits is approximately 100 bp.
1) Yes, the kit is compatible with automated instruments. However, the compatibility with specific bands of instruments needs confirmation from our technical team.
2) Buffer NT, Proteinase K, and Buffer LA should not be premixed.
Yes, supplier audits of ACROBiosystems' manufacturing facilities are acceptable. We welcome audits from our customers and partners as part of our commitment to transparency and quality assurance. To arrange an audit activity, please contact your local ACROBiosystems sales representative.
ACROBiosystems' resDetect™ Plasmid DNA Residual Detection Kit (Catalog Number: OPA-R009), is developed using a qPCR-based method, enabling precise quantification of residual plasmid DNA throughout the process of Cell and Gene Therapy (CGT) products. From intermediate stages to semi-finished and final products, this kit ensure the comprehensive monitoring and control of plasmid DNA impurities. Engineered for high sensitivity, consistency, and specificity, this kit effectively fulfills the rigorous quality control standards mandated for residual plasmid DNA in CGT therapies employing viral vectors.
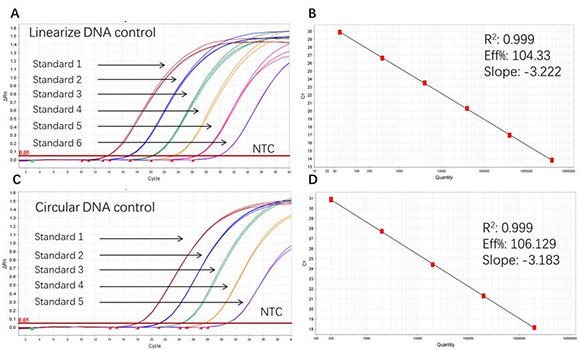
Figure 1. High sensitivity and broad dynamic range using the Plasmid resDNA Quantitation Kit. (A) Typical analysis results of obtained with Linearize DNA control Standard 1 (4×10⁶ copies/µL) to 6 (4×10 copies/µL). (B) The standard curve of the Linearize DNA control 10-fold dilution series. PCR efficiency should be 90-110%. (C) Typical analysis results of obtained with Circular DNA control Standard 1 (2×106 copies/µL) to 5 (2×10² copies/µL). (D) The standard curve of the Circular DNA control 10-fold dilution series. PCR efficiency should be 90-110%.
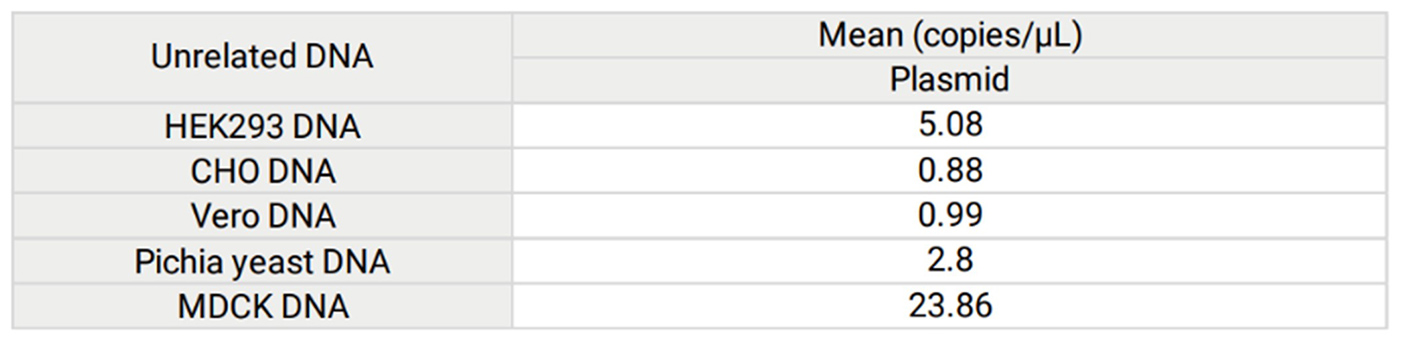
Figure 2. To evaluate the specificity of assay using Plasmid resDNA Quantitation Kit, no template samples spiked with unrelated DNA were tested. Results shown in the following Table. Unrelated DNA (HEK293, CHO, Vero, Pichia pastoris, and MDCK) were detected in assay, however the values of Mean were outside the range of standard curve (4×106 copies/µL to 4×10 copies/µL).
 Products
Products resDetect™ Plasmid resDNA Quantitation Kit (qPCR)
resDetect™ Plasmid resDNA Quantitation Kit (qPCR)
 resDetect™ E1A resDNA Quantitation Kit (qPCR)
resDetect™ E1A resDNA Quantitation Kit (qPCR)
 Resources
Resources Protocol——Plasmid resDNA Quantitation Kit (qPCR)
Protocol——Plasmid resDNA Quantitation Kit (qPCR)
 Brochure—resDetect™ CMC Manufacturing Process Residue Detection Solutions
Brochure—resDetect™ CMC Manufacturing Process Residue Detection Solutions
 FAQ
FAQThe primers are designed to target the ORI region (origin of replication) of commonly used plasmids, including psPAX2, pMD2.G, pHBLVTM, and many others. For specific inquiries, please contact our technical support team.
The amplicon size of this kit is approximately 100 bp.
Yes, ACROBiosystems welcomes supplier audits of our manufacturing facilities. To arrange an audit, please contact your local ACROBiosystems sales representative.
When analyzing qPCR data obtained with the Plasmid resDNA Quantiation kit, it is recommended to set the threshold line at 0.05 for optimal results.
In the manufacturing of viral vectors, the cell lines such as HEK293/HEK293T are commonly used to achieve the production goals. However, these cells contain oncogenes and viral sequences that can persist as risk elements in the final therapeutic products. Compliance with FDA CMC guidelines, specifically for cell-based gene therapies, requires the detection of these residual risk elements during quality research and control processes to ensure the safety and purity of therapeutic products.
To meet this critical requirement, we have developed resDetect™ quantitative residual detection kits tailored for E1A and SV40LTA DNA residues in CGT production, covering both cell and virus-based approaches. Utilizing the qPCR method, these kits enable rapid and reliable detection of residual risk element DNA content, with a quantitative limit of detection of 40 copies/μL. Typically, the entire experimental protocol can be completed within 4 hours, ensuring efficient and accurate assessment of product quality.

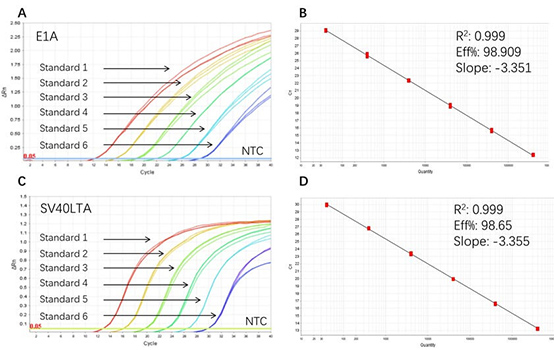
Figure 1. High sensitivity and broad dynamic range using the E1A&SV40LTA resDNA Quantitative Kit. (A) Typical analysis results of obtained with E1A DNA Control Standard 1 (4×10⁶ copies/µL) to 6 (4×10 copies/µL). (B) The standard curve of the E1A DNA Control 10-fold dilution series. PCR efficiency should be 90-110%. (C) Typical analysis results of obtained with SV40LTA DNA Control Standard 1 (4×10⁶ copies/µL) to 6 (4×10 copies/µL). (D) The standard curve of the SV40LTA DNA control 10-fold dilution series. PCR efficiency should be 90-110%.
 Products
Products resDetect™ E1A&SV40LTA resDNA Quantitation Kit (qPCR)
resDetect™ E1A&SV40LTA resDNA Quantitation Kit (qPCR)
 resDetect™ E1A resDNA Quantitation Kit (qPCR)
resDetect™ E1A resDNA Quantitation Kit (qPCR)
 FAQ
FAQThe amplicon size for resDetect™ residual viral oncogenes kits is approximately 100 bp.
When analyzing qPCR data obtained with this kit, it is recommended to set the threshold line at 0.05 for optimal results.
The plasmid standards are calibrated using digital PCR (dPCR) technology. This ensures accurate and precise quantification of the target residual DNA.
Yes, we welcome supplier audits of our manufacturing facilities. To arrange an audit, please contact your local ACROBiosystems sales representative.
In the manufacturing of immune cell therapies, key cell culture cytokines like, IL-2, IL-15, IL-7, and the monoclonal antibody, OKT3, play vital roles in driving immune cell proliferation and differentiation. However, residual levels of these raw/ancillary materials must meet strict quality standards outlined in regulatory guidelines such as USP<1043>, which emphasizes the development of robust residual detection methods to ensure consistent quality, efficacy, and safety of CGT product.
We offer a comprehensive array of resDetect™ assay kits for detecting residual cytokines and antibodies, renowned for their exceptional sensitivity, specificity, and overall quality. These kits are specifically designed to facilitate the precise detection of cytokine, growth factors and antibody residues during CMC process quality control, enabling robust monitoring and ensuring regulatory compliance.
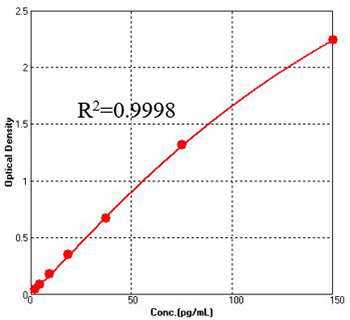
Figure 1. For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only. (Cat. No. CRS-A024)
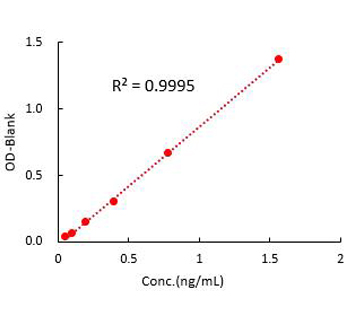
Figure 2. Detection of Monoclonal Anti-CD3 Antibody titer by indirect-ELISA Assay. Immobilized Human CD3E & CD3G can bind Anti-CD3 Antibody. Detection was performed using HRP-Goat anti-Mouse IgG with sensitivity of 0.098 ng/mL.
 Products
Products resDetect™Human Interleukin-15 (IL-15) ELISA Kit
resDetect™Human Interleukin-15 (IL-15) ELISA Kit
 resDetect™Human Interleukin-7 (IL-7) ELISA Kit
resDetect™Human Interleukin-7 (IL-7) ELISA Kit
 resDetect™Human Interleukin-21 (IL-21) ELISA Kit
resDetect™Human Interleukin-21 (IL-21) ELISA Kit
 Resources
Resources Protocol——resDetect™ Human Interleukin-15 (IL-15) ELISA Kit
Protocol——resDetect™ Human Interleukin-15 (IL-15) ELISA Kit
 Protocol——resDetect™ Anti-CD3 Antibody ELISA Kit
Protocol——resDetect™ Anti-CD3 Antibody ELISA Kit
 Brochure—resDetect™ CMC Manufacturing Process Residue Detection Solutions
Brochure—resDetect™ CMC Manufacturing Process Residue Detection Solutions
 FAQ
FAQExperimental data can vary across different users due to differences in several factors, including experimental conditions, instrumentation, and specific procedures followed. The “Typical Data” provided in the datasheet serves as a reference but may not precisely match your individual results.
A high Blank OD or background value could be caused by inadequate washing of the plate, incomplete drying, or the presence of residual liquid in the wells. Ensure that you follow the recommended washing and drying steps to minimize background signal.
After opening the kit, it is crucial to store each component under the specified conditions outlined in the instructions. Variations in experimental data can occur due to differences in laboratory environments, such as temperature or humidity, as well as slight deviations in procedures between different days.
Yes, components from different kits of the same batch number can be used interchangeably without compromising the assay performance. However, it is important to note that components from different batches should not be mixed, as batch-specific formulations may vary.
Stringent control of residual antibiotic levels is critical for upholding the safety and efficacy of cell and gene therapy (CGT) products. Commonly used antibiotics like kanamycin and gentamicin pose risks such as auditory nerve damage, nephrotoxicity, and allergic reactions. Regulatory bodies prioritize minimizing allergenic materials like β-lactam antibiotics (e.g., penicillin) in production processes.
We offer highly sensitive and specific antibiotic residue detection kits to support CGT manufacturers' compliance with regulatory standards. These user-friendly kits require minimal sample volumes and provide broad applicability across CGT production workflows. By enabling precise monitoring and control of antibiotic residues, our kits ensure product safety while mitigating potential adverse effects, ultimately safeguarding patients' well-being and fostering confidence in the integrity of CGT.

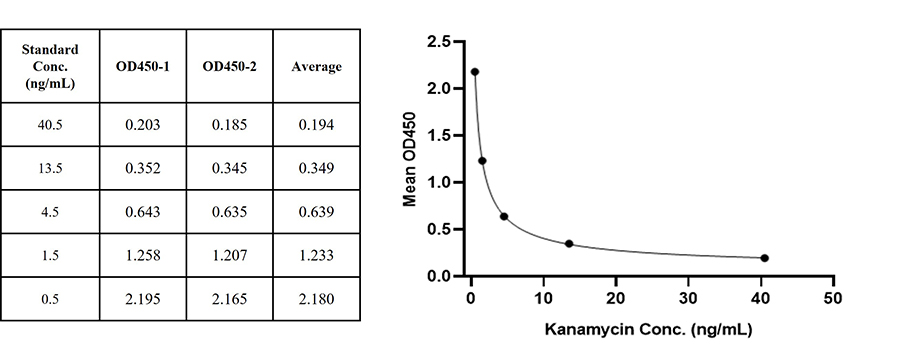
Figure 1. For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only. (Cat. No. RES-A004)
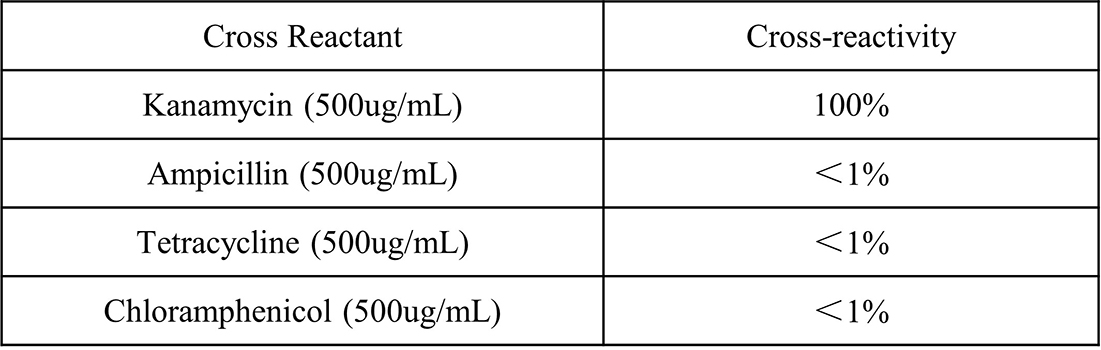
Figure 2. It has been verified that no significant cross-reactivity was observed when 500 μg/mL of ampicillin, tetracycline, and chloramphenicol were individually added to the sample diluent.
 FAQ
FAQGradient differences in the standard curve may result from errors in dilution, inaccurate pipetting, or incomplete plate washing. To ensure accuracy, follow dilution instructions precisely, calibrate pipettes, and verify washing cycles and volumes.
Faint or colorless results may stem from insufficient incubation time, incorrect temperature, or inadequate reagent volume. Refer to the manual, adhere strictly to recommended incubation protocols, ensure precise pipetting, and follow the correct reagent addition sequence.
Recovery rates may fall below specifications due to interference from buffers or the sample matrix. Perform serial dilutions to determine an appropriate matrix recovery dilution (MRD) factor without compromising assay performance.
This web search service is supported by Google Inc.