Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> VZV vaccines and drugs R&D solutions

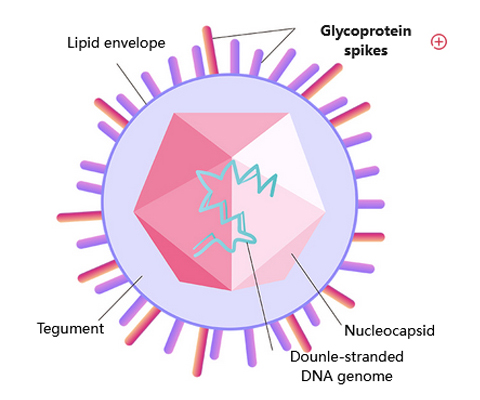
Current updates as to where we stand with eliminating varicella zoster virus (VZV):
![]() The first VZV vaccine, Zostavax, was developed by Merck and was approved for marketing in 2006.
The first VZV vaccine, Zostavax, was developed by Merck and was approved for marketing in 2006.
![]() Shingrix is a VZV subunit vaccine developed by GSK and was approved by the US FDA in October 2017. The improved efficacy and marketing of Shingrix, the Zostavax market share was quickly diminished and has been discontinued.
Shingrix is a VZV subunit vaccine developed by GSK and was approved by the US FDA in October 2017. The improved efficacy and marketing of Shingrix, the Zostavax market share was quickly diminished and has been discontinued.
![]() SKYZoster™ is a live vaccine was approved in South KMFDS in September 2017 based on results of clinical trials conducted in domestic and global institutions.
SKYZoster™ is a live vaccine was approved in South KMFDS in September 2017 based on results of clinical trials conducted in domestic and global institutions.
![]() In 2023, the live attenuated herpes zoster vaccine developed by Changchun Baker Biotechnology Co., Ltd. obtained the biological products batch certificate from the National Medical Products Administration.
In 2023, the live attenuated herpes zoster vaccine developed by Changchun Baker Biotechnology Co., Ltd. obtained the biological products batch certificate from the National Medical Products Administration.
| Product type | Molecule | Cat. No. | Product Description |
|---|
![]() Over 90% purity verified by SDS-PAGE;
Over 90% purity verified by SDS-PAGE;
![]() The binding activity of the antigen and its antibody was verified by ELISA with a linear range of 0.1-4ng/mL;
The binding activity of the antigen and its antibody was verified by ELISA with a linear range of 0.1-4ng/mL;
![]() The proteins are expressed in HEK293 cells, which makes sure the proteins'structure is closer to the natural conformation.
The proteins are expressed in HEK293 cells, which makes sure the proteins'structure is closer to the natural conformation.
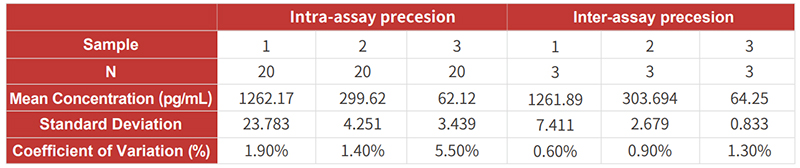
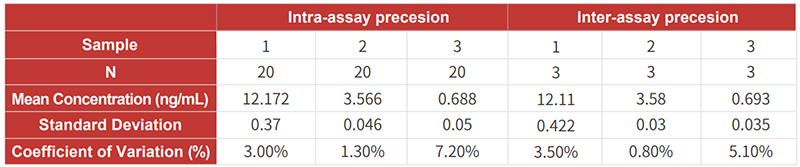
![]() High precision: CV values of intra- and inter-assay precision are <10%;
High precision: CV values of intra- and inter-assay precision are <10%;
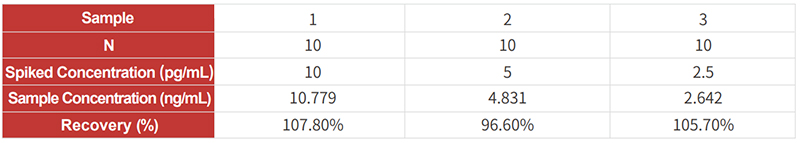
![]() High recovery rate: the recovery rate is within 80%-120%;
High recovery rate: the recovery rate is within 80%-120%;
![]() Provide kits for human and mouse sample detection, supporting the evaluation of humoral immunity in preclinical and early clinical stages.
Provide kits for human and mouse sample detection, supporting the evaluation of humoral immunity in preclinical and early clinical stages.
![]() High sensitivity: The sensitivity of the kit is over 39 pg/mL;
High sensitivity: The sensitivity of the kit is over 39 pg/mL;
![]() Wide detection range: The detection range of the kit is 39-1250pg/mL, which can meet the detection needs of different concentrations;
Wide detection range: The detection range of the kit is 39-1250pg/mL, which can meet the detection needs of different concentrations;
![]() High precision: CV values of intra- and inter-assay precision are <10%.
High precision: CV values of intra- and inter-assay precision are <10%.
![]() High sensitivity and specificity, can accurately detect gE protein in samples;
High sensitivity and specificity, can accurately detect gE protein in samples;
![]() Recombinant expression in HEK293 cells with good batch-to-batch stability;
Recombinant expression in HEK293 cells with good batch-to-batch stability;
![]() Can be used for gE protein detection or as an antibody standard.
Can be used for gE protein detection or as an antibody standard.

Varicella zoster virus (strain Oka vaccine) Envelope Glycoprotein E (gE), His Tag on SDS-PAGE under non-reducing (NR) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90%.
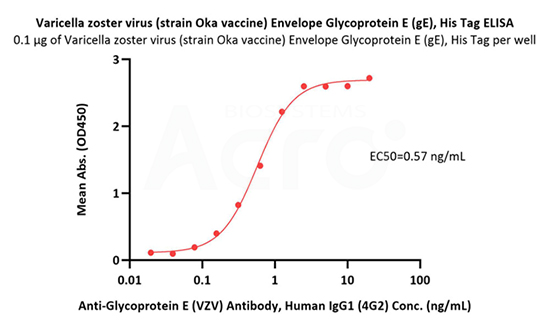
Immobilized Varicella zoster virus (strain Oka vaccine) Envelope Glycoprotein E (gE), His Tag (Cat. No. GLE-V52H3) at 1 μg/mL (100 μL/well) can bind Anti-Glycoprotein E (VZV) Antibody, Human IgG1 (4G2) with a linear range of 0.02-1 ng/mL (QC tested).
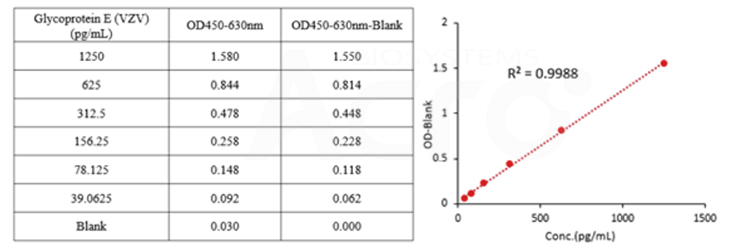
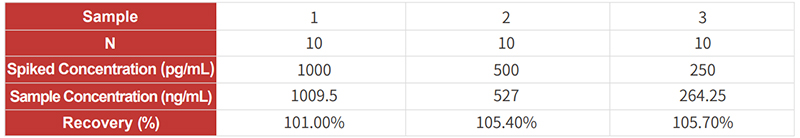
The detection range of Cat. No. RAS-A102 is 39-1250pg/mL, and the sensitivity is 39pg/mL.
The LOQ for kit RAS-A102 is 78 pg/mL, and the LOD is 39 pg/mL.
Both the capture and detection antibodies used in RAS-A185 are monoclonal antibodies.
The RAS-A185 and RAS-A102 kits differ in sensitivity and colorimetric methods. The RAS-A185 uses HRP-Anti-Glycoprotein E (VZV) antibody for direct color development, while RAS-A102 employs Biotin-Anti-Glycoprotein E (VZV) antibody plus Streptavidin-HRP for color development. Both can be used for quantitative detection of VZV gE protein, but RAS-A102 has higher sensitivity, allowing you to choose the appropriate kit based on protein expression levels.
This web search service is supported by Google Inc.