 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| AHG-Y69 | Mouse | Anti-Human IgG Antibody-Acridinium ester (MALS verified) |
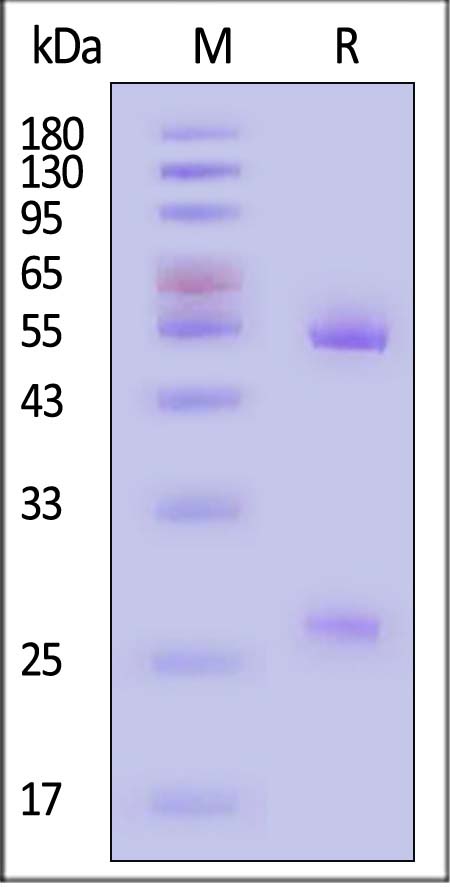
|
||
| IGG-LY69 | Mouse | HRP conjugated Anti-Human-IgG-Fc Antibody (6F11C8), mAb |

Immobilized Human TNF-alpha Protein, His Tag (Cat. No. TNA-H5228) at 2 μg/mL (100 μL/well) can bind Human Monoclonal Anti-TNF-alpha antibody, Human IgG1 (16H5) (Cat. No. TNA-AM494) when detected by HRP conjugated Anti-Human-IgG-Fc Antibody (6F11C8),mAb (Cat. No. IGG-LY69) dilute at 1:10000 (0.0842μg/ml) (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Imlifidase | Mac-1 | Approved | Hansamed Inc | Idefirix | EU | Rejection of renal transplantation | Hansa Biopharma Ab | 2020-08-25 | Rejection of renal transplantation; Kidney Diseases; Purpura, Thrombotic Thrombocytopenic; Rejection of organ transplantation; Renal Insufficiency, Chronic; Guillain-Barre Syndrome; Kidney Failure, Chronic | Details |
| Imlifidase | Mac-1 | Approved | Hansamed Inc | Idefirix | EU | Rejection of renal transplantation | Hansa Biopharma Ab | 2020-08-25 | Rejection of renal transplantation; Kidney Diseases; Purpura, Thrombotic Thrombocytopenic; Rejection of organ transplantation; Renal Insufficiency, Chronic; Guillain-Barre Syndrome; Kidney Failure, Chronic | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| AT-02 | AT-02 | Phase 2 Clinical | Attralus Inc | Immunoglobulin Light-chain Amyloidosis; Amyloidosis | Details |
| Anti-CD33/anti-FcgammaRI bispecific antibody (UCSD) | Phase 1 Clinical | University Of California San Diego | Leukemia, Myeloid, Acute | Details | |
| AT-02 | AT-02 | Phase 2 Clinical | Attralus Inc | Immunoglobulin Light-chain Amyloidosis; Amyloidosis | Details |
| Anti-CD33/anti-FcgammaRI bispecific antibody (UCSD) | Phase 1 Clinical | University Of California San Diego | Leukemia, Myeloid, Acute | Details |
This web search service is supported by Google Inc.