 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> 抗イディオタイプ抗体
抗イディオタイプ抗体は、別の抗体の可変領域を認識し、特異的な結合を生成する抗体です。 抗イディオタイプ抗体は、薬剤開発に広く使用されています。免疫原性分析の重要な基準として使用でき、またinvivoでの抗体薬剤レベルを特異的に検出することもできるため、薬物動態研究における重要な試薬です。
前臨床/臨床免疫原性とPK解析をサポートするために、ACROBiosystemsは、様々な高親和性、高特異性の抗イディオタイプ抗体を開発しました。さらに、創薬プロセスを加速できるように、抗イディオタイプ抗体を使用する試験に適用のプロトコルも提供しております。弊社のパイプラインには、adalim*mab, Ritux*mab, Cetux*mab, Trastuz*mab, and Bevaciz*mab などのターゲットも含まれています。
| Molecule | Cat. No. | Antigen | Neutralizing Activity | Application |
|---|---|---|---|---|
| Adalimu*ab | ADB-Y19 | Anti-Adalimu*ab Antibodies (AY19) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y10 | Anti-Bevacizu*ab Antibodies (AY10) (MALS verified, recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y12 | Anti-Bevacizu*ab Antibodies (AY12) (recommended for neutralizing assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y9 | Anti-Bevacizu*ab Antibodies (AY9) (recommended for ADA assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-BY13 | Biotinylated Anti-Bevacizu*ab Antibodies (AY13) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA;Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y27 | Anti-Cetuxi*ab Antibodies (AY27) (recommended for ADA assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y31 | Anti-Cetuxi*ab Antibodies (AY31) (Non-Neutralizing) | Non-Neutralizing Antibody | ADA assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y28 | Anti-Cetuxi*ab Antibodies (AY28) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-BY31 | Biotinylated Anti-Cetuxi*ab Antibodies (AY31) (recommended for PK/PD) | Non-Neutralizing Antibody | PK bridging ELISA; Indirect ELISA |
| Rituxi*ab | RIB-Y36 | Anti-Rituxi*ab Antibodies (AY36) (recommended for ADA assay) | Neutralizing Antibody | ADA assay;Neutralizing assay; Indirect ELISA |
| Rituxi*ab | RIB-Y37 | Anti-Rituxi*ab Antibodies (AY37) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA;Neutralizing assay;Indirect ELISA |
| Rituxi*ab | RIB-FY35c | FITC-Labeled Anti-Rituxi*ab Antibodies, Mouse IgG1 | Neutralizing Antibody | ADA assay;Neutralizing assay; Indirect ELISA |
| Trastuzu*ab | TRB-Y5b | Anti-Trastuzu*ab Antibodies (AY5b) (recommended for PK/PD) | Non-Neutralizing Antibody | Neutralizing Antibody |
| Trastuzu*ab | TRB-Y1b | Anti-Trastuzu*ab Antibodies (AY1b) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA; Neutralizing assay; Indirect ELISA |
ほとんど全ての生物学的製品は特定の抗薬物抗体(ADA)を誘発します。ADAの出現によって、薬物の有効性が低下、または副作用を引き起こされる可能性があります。そのため、分子の免疫原性を評価し、実験結果を臨床応用に関連付けるには、前臨床研究と臨床研究において、ADA応答を効果的に評価できる実験法が必要となります。
キャリブレーション標準としてのADAがないため、ADAの測定は通常非定量的実験です。また、ADA測定用のポジティブコントロールとして、モノクローナル/マルチクローナル抗体を開発することは、非常に時間がかかります。この問題を解決するために、ACROBiosystemsは、ADAアッセイ用に、標準化された抗薬物抗体をたくさん開発しました。
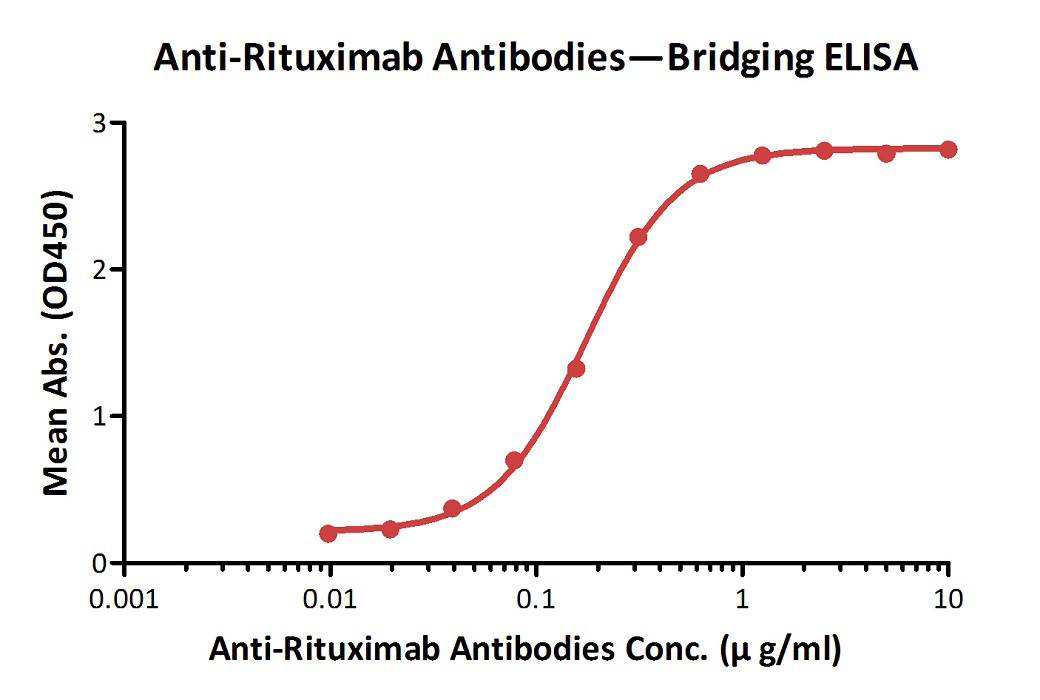
Anti-Ritux*mab Antibodies bridging ELISA for Anti-Drug Antibody (ADA) assay development. Immobilized Ritux*mab at 1 µg/ml, added increasing concentrations of Anti-Ritux*mab Antibodies (Cat. No. RIB-Y36, 10% human serum) and then added biotinylated Ritux*mab at 2 µg/ml. Detection was performed using HRP-conjugated streptavidin with a sensitivity of 9.7 ng/mL.
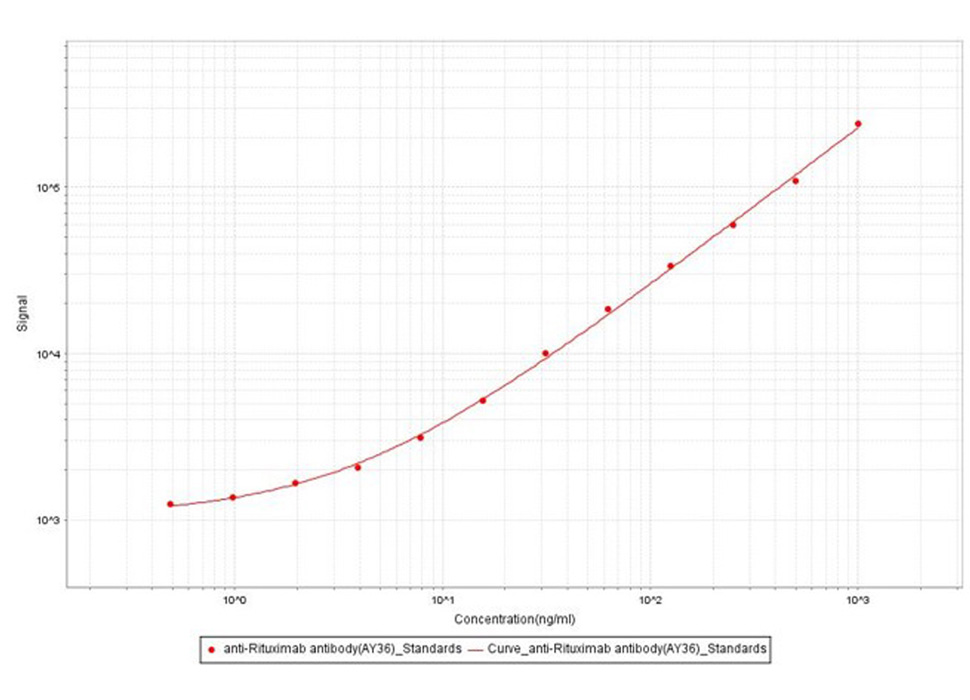
Anti-Ritux*mab Antibodies bridging MSD for Anti-Drug Antibody (ADA) assay development. Added the mix solution (biotinylated Ritux*mab at 5 µg/mL, SULFO-Ritux*mab at 5Nµg/mL and increasing concentrations of Anti-Ritux*mab Antibodies (Cat. No. RIB-Y36, 100% human serum). Detection was performed using MSD Assay with a sensitivity of 0.97 ng/mL.
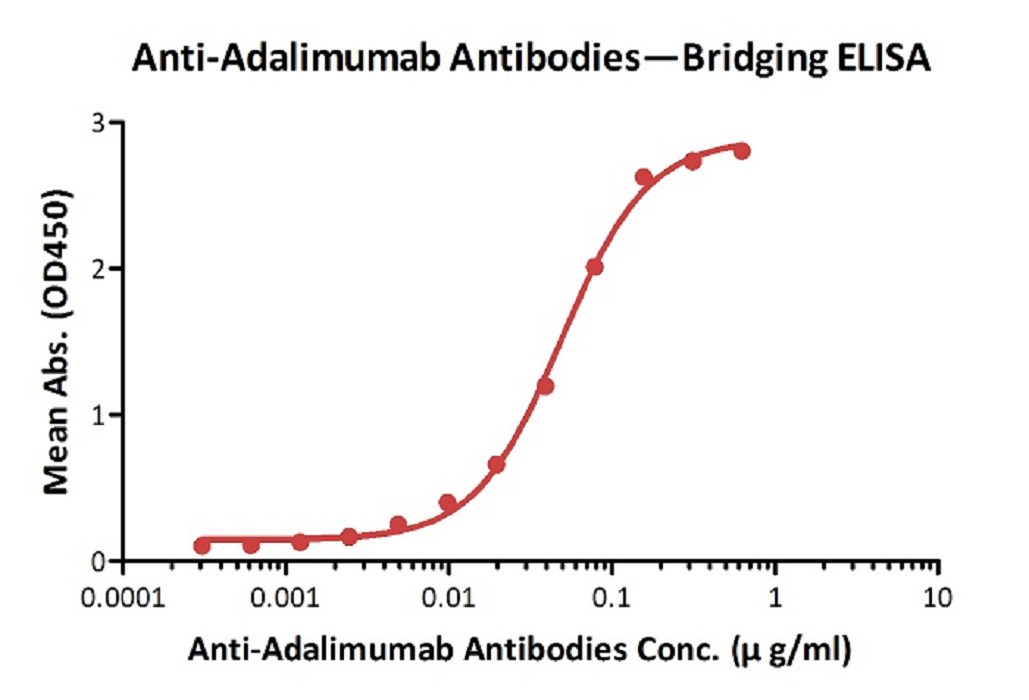
Anti-Adalim*mab Antibodies bridging ELISA for Anti-Drug Antibody (ADA) assay development. Immobilized adalim*mab at 1 µg/ml, add increasing concentrations of Anti-Adalim*mab Antibodies (Cat. No. ADB-Y19, 10% human serum) and then add biotinylated adalim*mab at 5 µg/ml. Detection was performed using HRP-conjugated streptavidin with a sensitivity of 0.6 ng/mL.
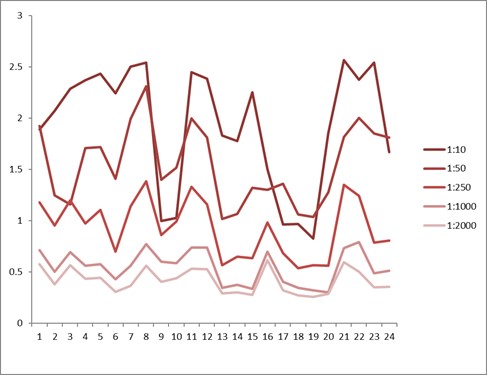
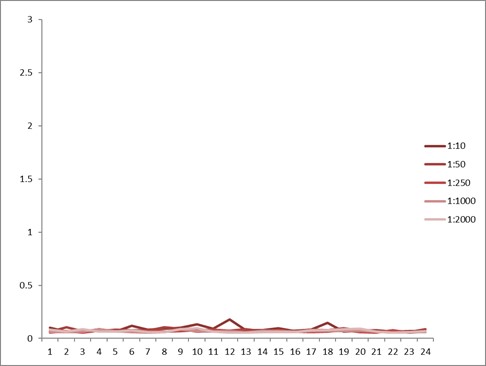
Comparison between anti-idiotypic capture ELISA and anti-idiotypic bridging ELISA for Ritux*mab detection in patient samples. Left: anti-idiotypic capture ELISA; Right: anti-idiotypic bridging ELISA.
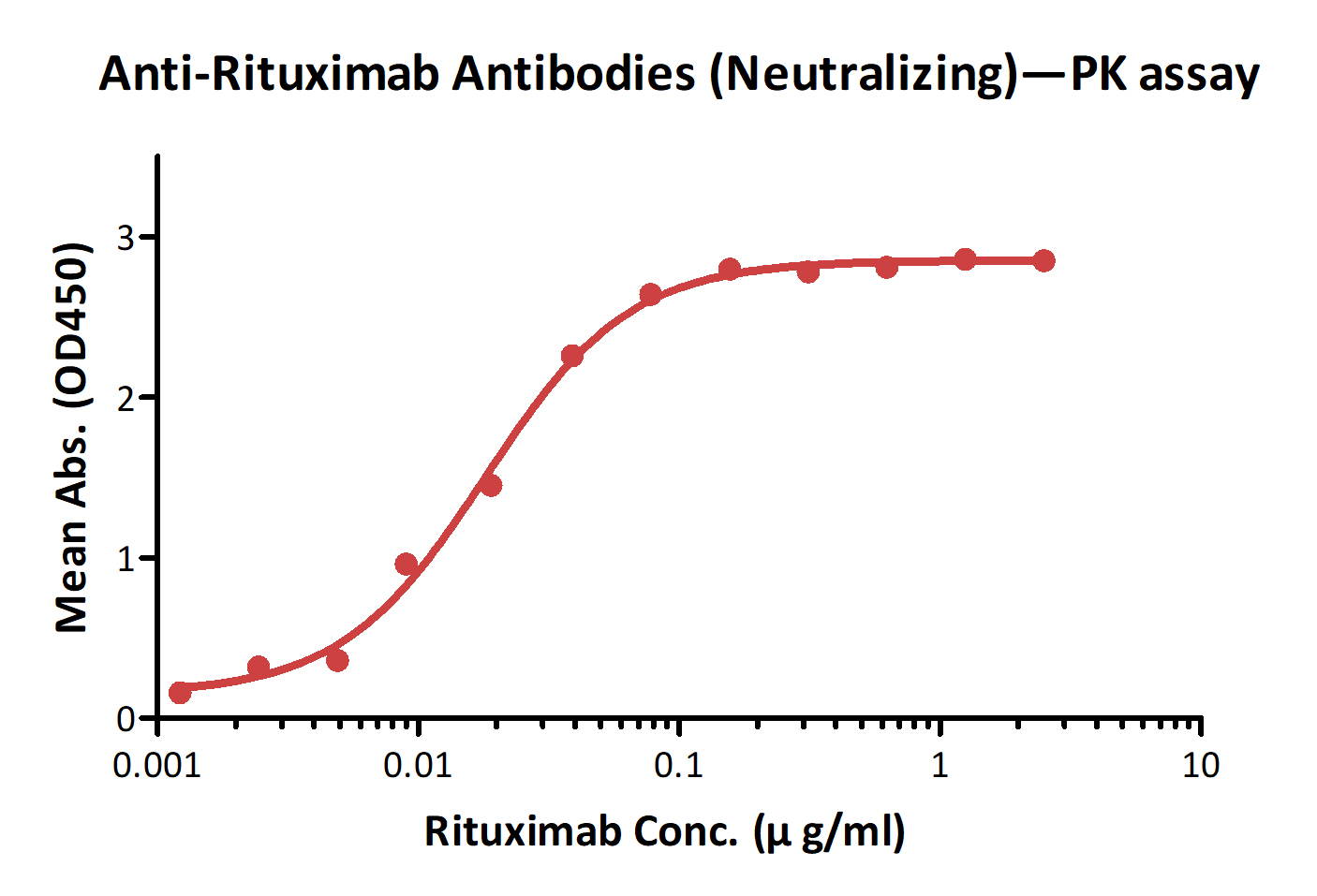
Detection of Ritux*mab by bridging ELISA in serum. Immobilized Anti-Ritux*mab Antibodies (Cat. No. RIB-Y37) at 2 μg/ml, added increasing concentrations of Ritux*mab (10% human serum) and then added biotinylated Anti-Ritux*mab Antibodies (Cat. No. RIB-BY35) at 1 μg/ml. Detection was performed using HRP-conjugated streptavidin with a sensitivity of 1 ng/ml.
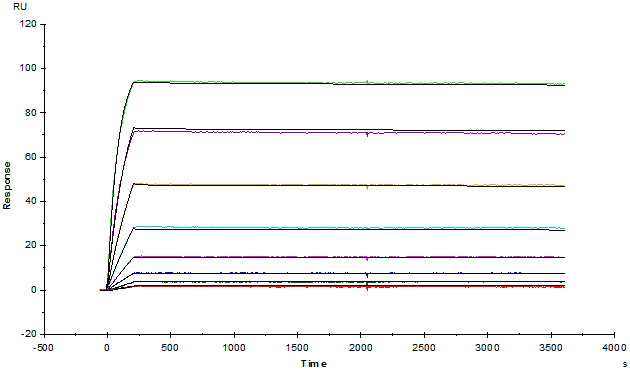
Anti-Adalim*mab Antibodies (mouse IgG1, Cat. No. ADB-Y19) captured on CM5 chip via anti-mouse antibodies surface, can bind human adalim*mab with an affinity constant of 1.36 pM.
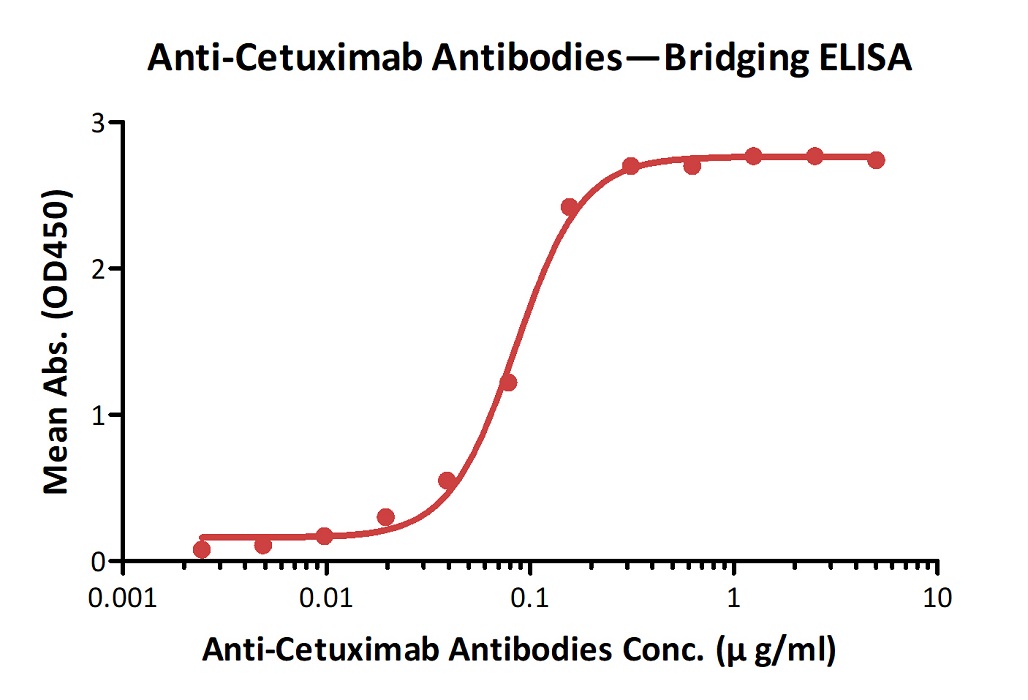
Demonstration of the specificity of Anti-Cetux*mab Antibodies (Cat. No. CEB-Y28) to the Cetux*mab.
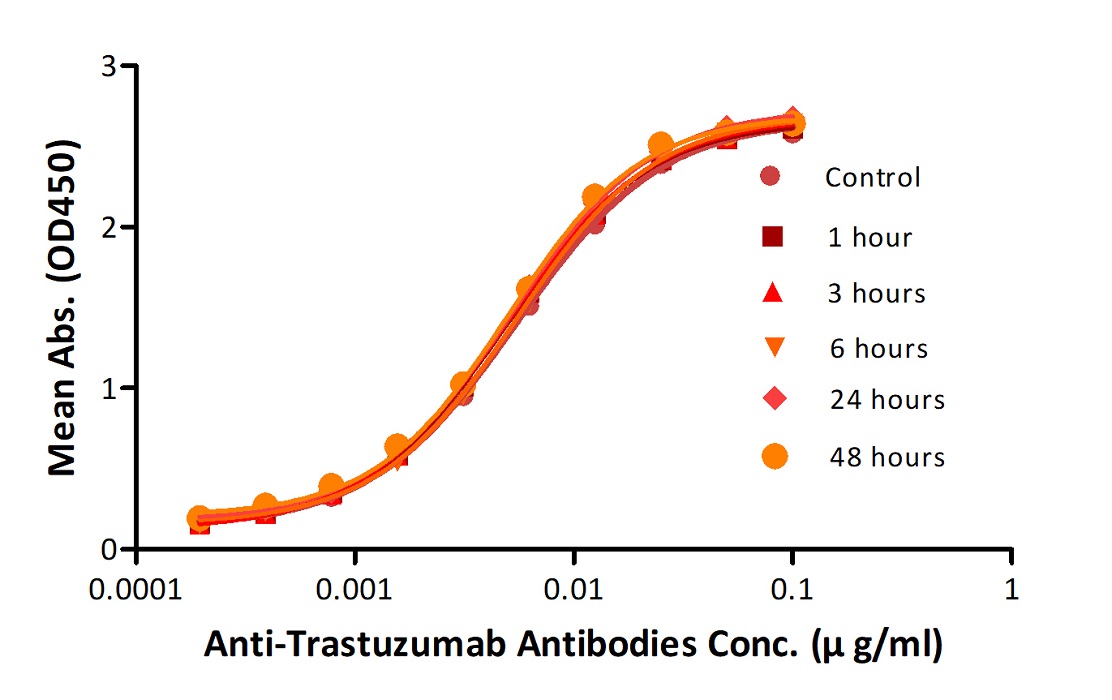
Reconstituted Anti-Trastuz*mab Antibodies were diluted to 0.4 mg/ml, aliquoted and placed at 37°C. Aliquots were removed from 37°C at every time point and placed at 4°C along with the control. No significant loss of activity was observed.
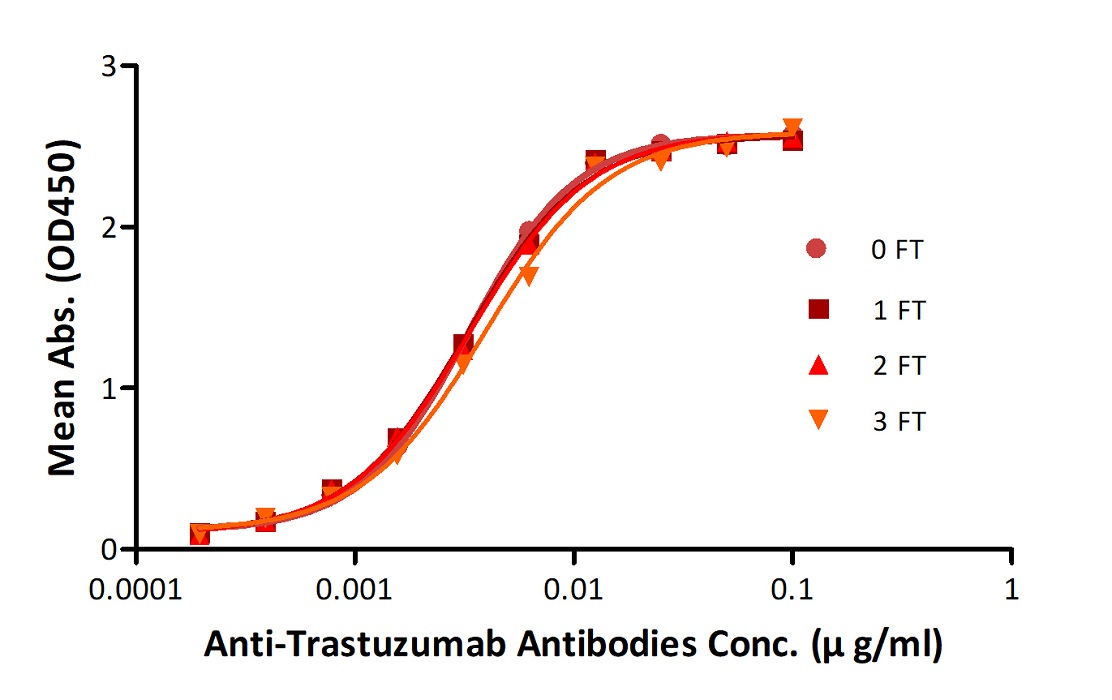
Anti-Trastuz*mab Antibodies were subjected to the indicated number of freeze-thaw cycles (FT). No significant loss of activity was observed.
This web search service is supported by Google Inc.