





























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Solutions for RSV vaccine and drug development

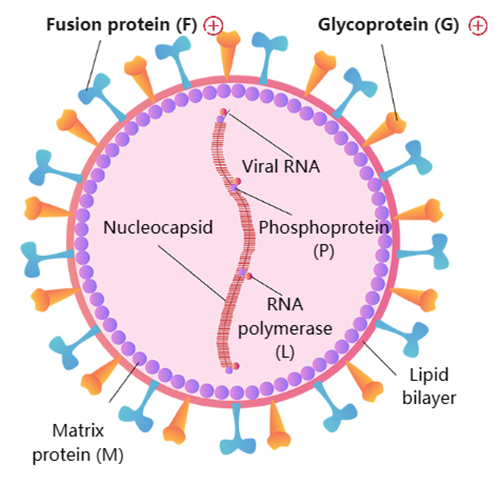
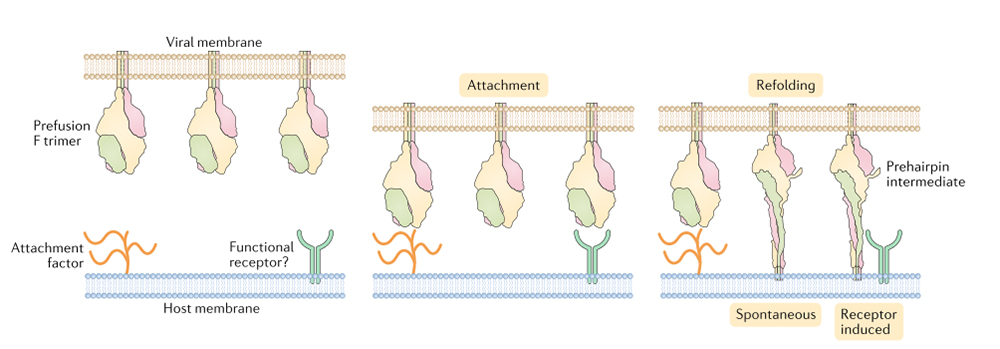
(10.1038/s41579-019-0149-x)
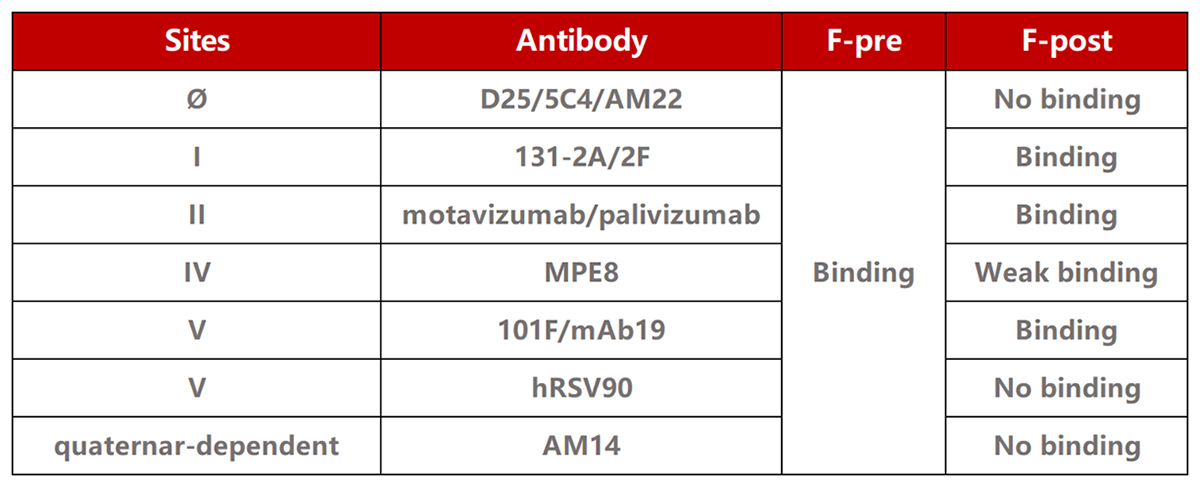
| Product type | Molecule | Cat. No. | Product Description |
|---|
![]() Provide native, structurally verified trimeric Pre-F/Post-F/G proteins, Pre-F/Post-F broadly & specific antibodies and ELISA kits;
Provide native, structurally verified trimeric Pre-F/Post-F/G proteins, Pre-F/Post-F broadly & specific antibodies and ELISA kits;
![]() Over 90% purity verified by SDS-PAGE and SEC-MALS
Over 90% purity verified by SDS-PAGE and SEC-MALS
![]() Binding activity of Pre-F and Post-F proteins verified through ELISA with their respective specific antibodies.
Binding activity of Pre-F and Post-F proteins verified through ELISA with their respective specific antibodies.
![]() High immunogenicity: native trimeric antigens can induce higher neutralizing antibody titers.
High immunogenicity: native trimeric antigens can induce higher neutralizing antibody titers.
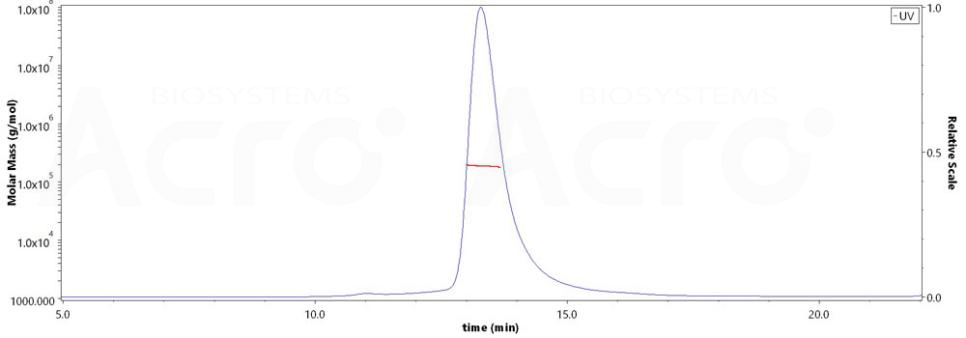
The purity of HRSV (A) Fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H7) is more than 90% and the molecular weight of this protein is around 175-200 kDa verified by SEC-MALS.
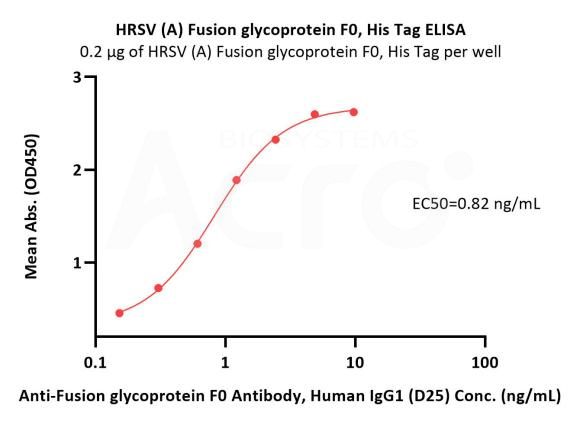
Immobilized HRSV (A) Fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H7) at 2 μg/mL (100 μL/well) can bind Anti-Fusion glycoprotein F0 Antibody, Human IgG1 (D25) with a linear range of 0.2-1 ng/mL (QC tested).
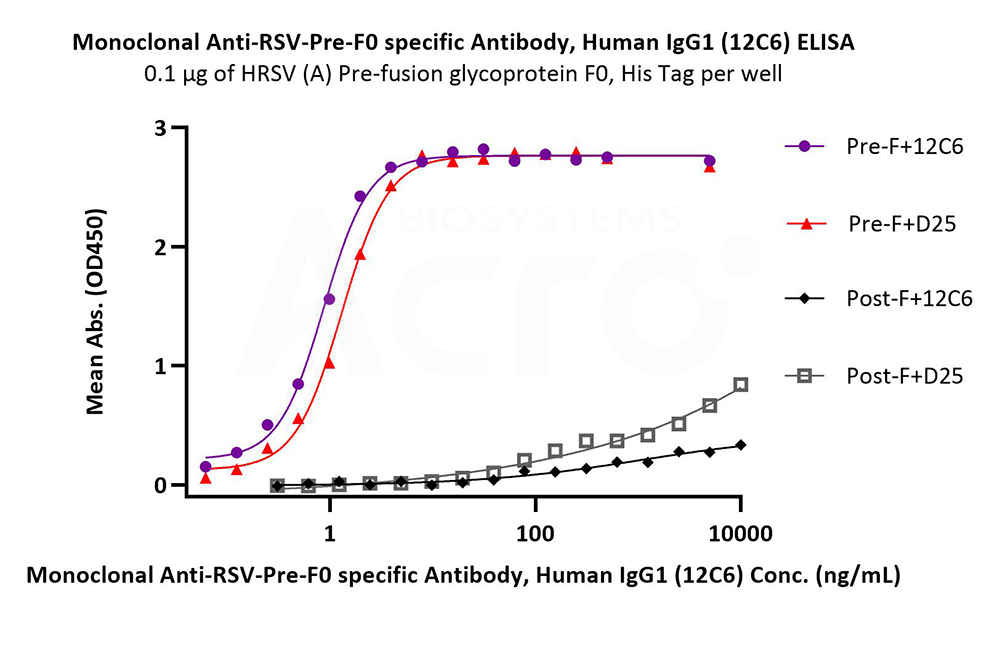
Immobilized HRSV (A) Pre-fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H7) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-RSV-Pre-F0 specific Antibody, Human IgG1 (12C6) (Cat. No. RS0-S286) with a linear range of 0.2-2 ng/mL. HRSV (A) Post-fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H6) is verified not recoginized by Monoclonal Anti-RSV-Pre-F0 specific Antibody, Human IgG1 (12C6) (Cat. No. RS0-S286) in low concentration (QC tested).
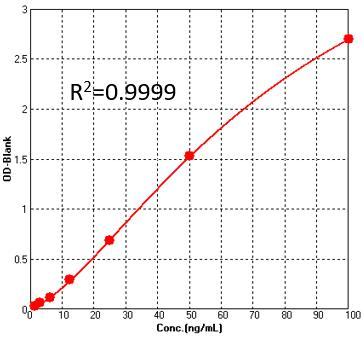
Detection of Pre-Fusion glycoprotein F0 (RSV) by sandwich-ELISA Assay.Immobilized Anti-Pre-Fusion glycoprotein F0 (RSV) Antibody can bind Pre-Fusion glycoprotein F0 (RSV). Detection was performed using HRP-Anti-Pre-Fusion glycoprotein F0 (RSV) Antibody with sensitivity of 3.1 ng/mL (QC tested).
> Click to see more Core reagents for infectious disease research
This web search service is supported by Google Inc.







