 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| NEE-MY2069 | Mouse | Monoclonal Anti-Influenza A virus NA (H1N1) (Victoria/2570/2019) Antibody, Human IgG1 (8E5) (MALS verified) |
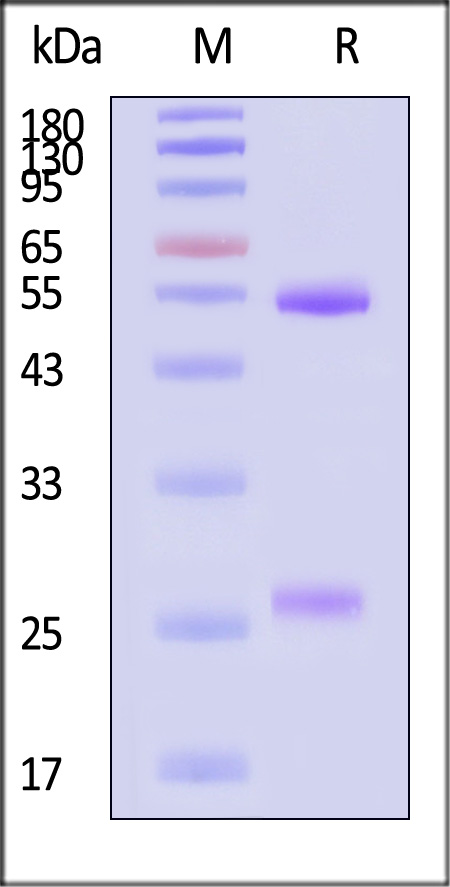
|
||
| NEE-V524x | Influenza A virus (A/Croatia/10136RV/2023) & (A/District of Columbia/27/2023) | Influenza A virus (A/Croatia/10136RV/2023) & (A/District of Columbia/27/2023) NA(H3N2) Protein, His Tag |
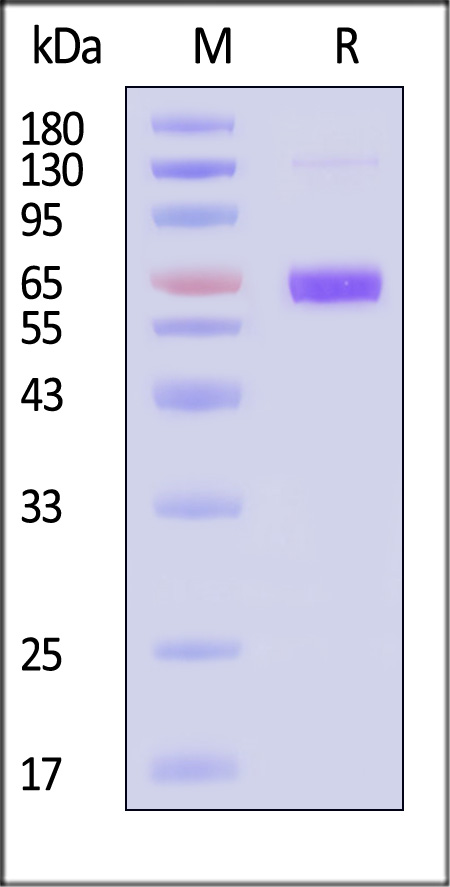
|
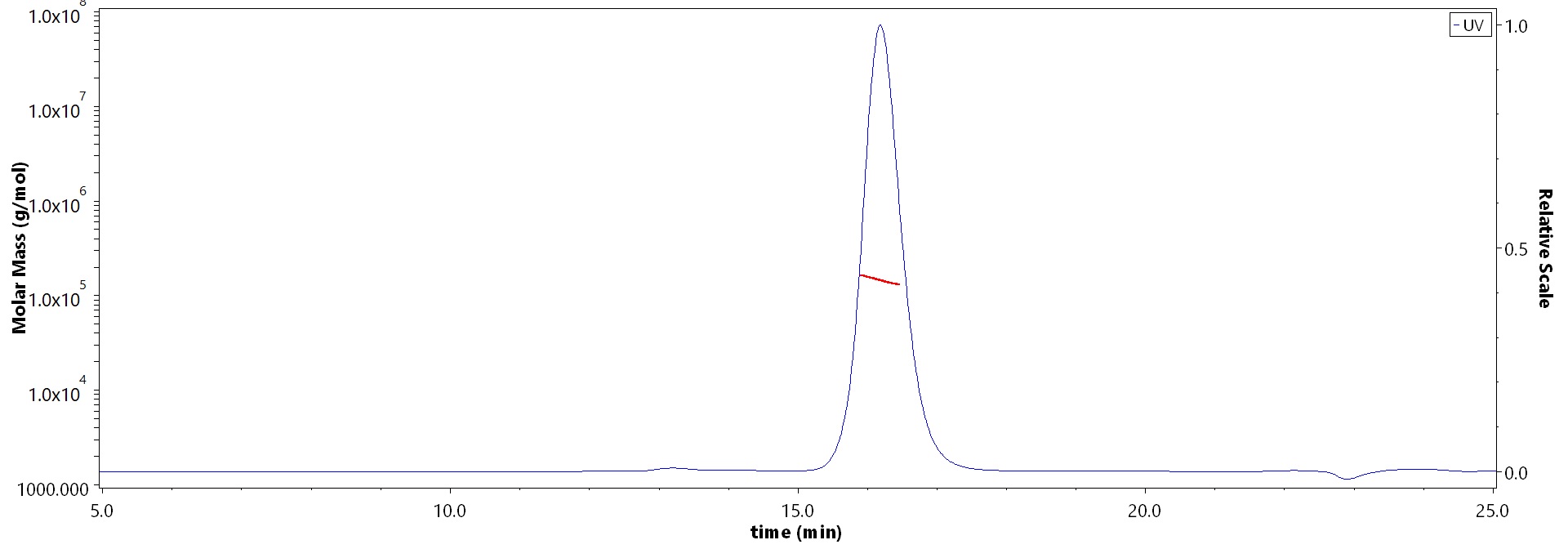
The purity of Monoclonal Anti-Influenza A virus NA (H1N1) (Victoria/2570/2019) Antibody, Human IgG1 (8E5) (Cat. No. NEE-MY2069) is more than 90% and the molecular weight of this protein is around 135-160 kDa verified by SEC-MALS.
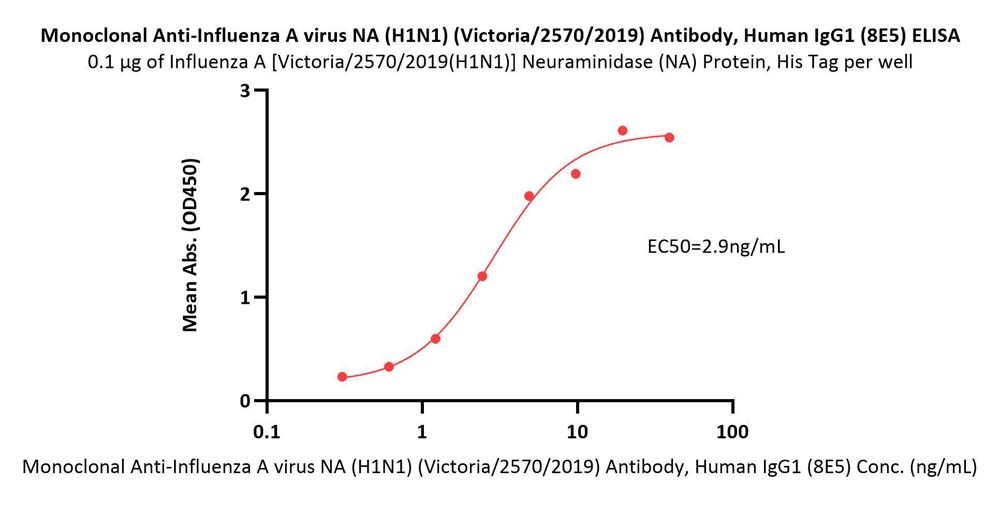
Immobilized Influenza A [Victoria/2570/2019(H1N1)] Neuraminidase (NA) Protein, His Tag (Cat. No. NEE-V524e) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-Influenza A virus NA (H1N1) (Victoria/2570/2019) Antibody, Human IgG1 (8E5) (Cat. No. NEE-MY2069) with a linear range of 0.3-5 ng/mL (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Laninamivir Octanoate Hydrate | CS-8958; R-118958 | Approved | Daiichi Sankyo Co Ltd | Inavir | Japan | Influenza B virus infection; Influenza A virus infections; Influenza, Human | null | 2010-09-10 | Influenza A virus infections; Influenza, Human; Asthma; Influenza B virus infection | Details |
| Zanamivir | GG-167; GR-121167; GR-121167X; 4-Guanidino-Neu5Ac2en | Approved | Glaxosmithkline Plc | 依乐韦, Relenza | United States | Influenza, Human | Glaxosmithkline Plc | 1999-07-26 | Orthomyxoviridae Infections; Diabetes Mellitus, Type 2; Influenza, Human; Arthralgia; Dengue | Details |
| Oseltamivir Phosphate | Ro-64-0796; GS-4104; RO-640796; GS-4104-002; EN-241104; RO-640796/002; GS-4104/002 | Approved | F. Hoffmann-La Roche Ltd | Tamiflu, Hanmi Flu, 达菲 | Switzerland | Influenza, Human | F. Hoffmann-La Roche Ltd | 1999-09-21 | Purpura, Thrombocytopenic, Idiopathic; Influenza A virus infections; Influenza, Human; Influenza in Birds; Obesity; Kidney Failure, Chronic | Details |
| Peramivir Hydrate | JNJ-2; BCX-1812; S-021812; RWJ-270201 | Approved | Biocryst Pharmaceuticals Inc | 力纬, Alpivab, Rapivab, Rapiacta, Peramiflu | Japan | Influenza, Human | null | 2010-01-13 | Cough; Headache; Myalgia; Musculoskeletal Pain; Fatigue; Influenza A virus infections; Influenza, Human; Fever; Arthralgia; Nasal Obstruction; Influenza B virus infection; Pharyngitis | Details |
| Laninamivir Octanoate Hydrate | CS-8958; R-118958 | Approved | Daiichi Sankyo Co Ltd | Inavir | Japan | Influenza B virus infection; Influenza A virus infections; Influenza, Human | null | 2010-09-10 | Influenza A virus infections; Influenza, Human; Asthma; Influenza B virus infection | Details |
| Zanamivir | GG-167; GR-121167; GR-121167X; 4-Guanidino-Neu5Ac2en | Approved | Glaxosmithkline Plc | 依乐韦, Relenza | United States | Influenza, Human | Glaxosmithkline Plc | 1999-07-26 | Orthomyxoviridae Infections; Diabetes Mellitus, Type 2; Influenza, Human; Arthralgia; Dengue | Details |
| Oseltamivir Phosphate | Ro-64-0796; GS-4104; RO-640796; GS-4104-002; EN-241104; RO-640796/002; GS-4104/002 | Approved | F. Hoffmann-La Roche Ltd | Tamiflu, Hanmi Flu, 达菲 | Switzerland | Influenza, Human | F. Hoffmann-La Roche Ltd | 1999-09-21 | Purpura, Thrombocytopenic, Idiopathic; Influenza A virus infections; Influenza, Human; Influenza in Birds; Obesity; Kidney Failure, Chronic | Details |
| Peramivir Hydrate | JNJ-2; BCX-1812; S-021812; RWJ-270201 | Approved | Biocryst Pharmaceuticals Inc | 力纬, Alpivab, Rapivab, Rapiacta, Peramiflu | Japan | Influenza, Human | null | 2010-01-13 | Cough; Headache; Myalgia; Musculoskeletal Pain; Fatigue; Influenza A virus infections; Influenza, Human; Fever; Arthralgia; Nasal Obstruction; Influenza B virus infection; Pharyngitis | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| AV-5080 | AV-5080 | Phase 3 Clinical | Viriom Inc | Influenza, Human | Details |
| HNC042 | HNC-042 | Phase 1 Clinical | Guangzhou Henovcom Bioscience Co Ltd | Influenza A virus infections; Influenza, Human; Influenza B virus infection | Details |
| Oseltamivir/Pimodivir | Phase 1 Clinical | Johnson & Johnson | Influenza A virus infections | Details | |
| Oseltamivir orally disintegrating tablets | HY-100209 | Phase 1 Clinical | Hefei Industrial Pharmaceutical Co Ltd | Influenza A virus infections; Influenza B virus infection | Details |
| AV-5080 | AV-5080 | Phase 3 Clinical | Viriom Inc | Influenza, Human | Details |
| HNC042 | HNC-042 | Phase 1 Clinical | Guangzhou Henovcom Bioscience Co Ltd | Influenza A virus infections; Influenza, Human; Influenza B virus infection | Details |
| Oseltamivir/Pimodivir | Phase 1 Clinical | Johnson & Johnson | Influenza A virus infections | Details | |
| Oseltamivir orally disintegrating tablets | HY-100209 | Phase 1 Clinical | Hefei Industrial Pharmaceutical Co Ltd | Influenza A virus infections; Influenza B virus infection | Details |
This web search service is supported by Google Inc.