





























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Insights > High-Quality SARS-CoV-2 Proteins are Essential for Sensitive and Accurate Diagnosis of COVID-19 The coronavirus pandemic has changed life as we know it and, as of September20th, has led to over 30 million people catching the disease and around 1000,000 deaths worldwide.1 The characteristics of the virus – an incubation period of up to 14 days, viral transmission during the presymptomatic phase, and a small asymptomatic population – along with the slow mobilization of diagnostic testing have resulted in countries imposing national lockdowns to limit the spread of the virus. 1,2
At the time of writing, these actions have managed to lower the number of cases and reduce the reproduction number, so many countries are lifting their lockdown restrictions. However, as there is currently no vaccine, lifting these restrictions may lead to new outbreaks, a situation that has already been observed in Germany.3 Therefore, accurate diagnosis of SARS-CoV-2 is essential to identify new outbreaks, implement timely quarantine, limit the spread of infection, and ultimately eliminate the virus.4,5
Diagnostic Tests for COVID-19
There are three main types of diagnostic tests to identify SARS-CoV-2: the molecular test, antibody, and antigen test. Both the molecular and antigen test identify the presence of an active coronavirus, while the antibody test can determine whether a patient has previously been infected with the disease.11 Due to the body’s immunological response the antibody tests have been shown to diagnose patients up to 35 days after the onset of COVID-19 symptoms.7 A comparison of these three tests are shown in the table below.
The problem with many established testing methodologies, such as reverse transcription-polymerase chain reactions (RT-PCR) which use an RNA template to detect SARS-CoV-2 genes, is that they are relatively slow and inconvenient. By contrast, antibody and antigen detection of COVID-19 infection is rapid, enabling faster diagnostics with some kits approaching instant detection.11
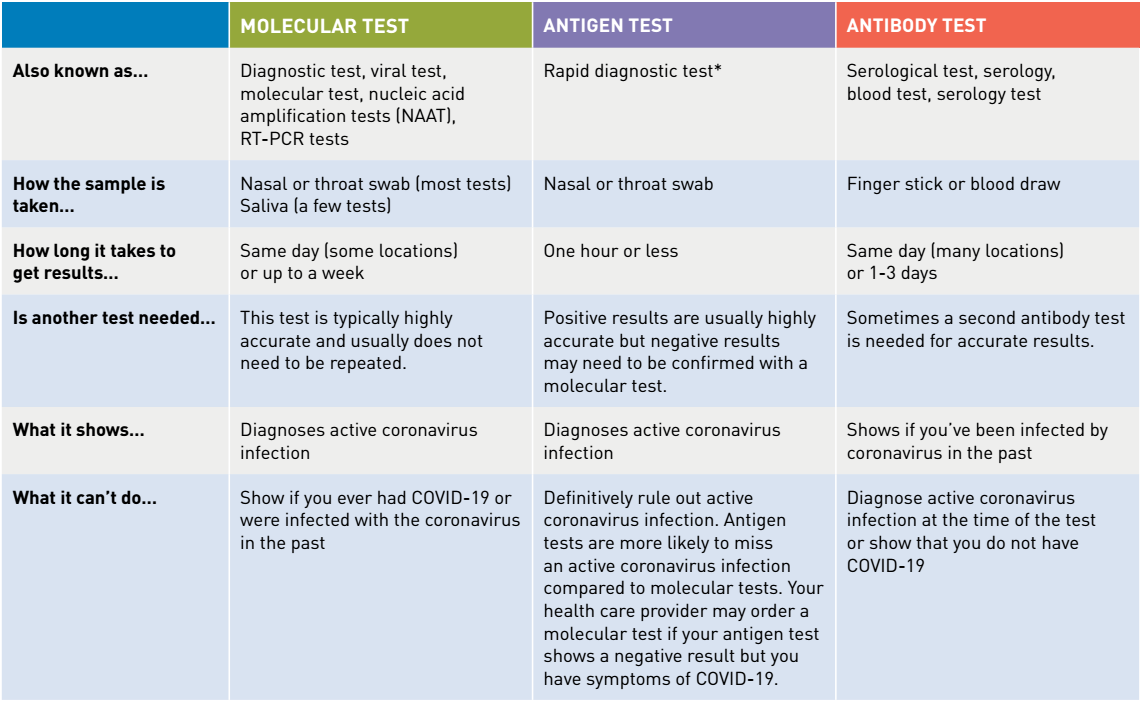
Source: https://www.fda.gov/media/138094/download
The Critical Role of Antigens and Antibodies in SARS-CoV-2 Rapid Diagnosis
As of August 28th, 194 diagnostic tests had received emergency use authorization from the FDA using three different detection methods, including 40 antibody tests and 4 antigen tests. 4,6,8 However, the combination of the urgent demand and relaxation of FDA guidelines led to some tests showing poor performance.7,8
One of the main interferences of immunoassay performance is cross-reactivity. Indeed, the two main proteins that are being used in SARS-CoV-2 serological tests – the spike (S) glycoprotein and the nucleocapsid (N) protein – contain immunogenic domains that are conserved in strains of other β-coronaviruses, some of which are known to cause the common cold.2 Therefore, highly specific antibody and antigen reagents are essential, and kit testing with high-purity control antibodies to ensure accuracy should be performed to avoid erroneous results.9,15
Zhao et al. used ACROBiosystems biotin-labeled S1 protein to develop a COVID-19 chemiluminescent immunoassay. By assessing the total antibody, rather than immunoglobulin (Ig)G or IgM concentration, the test achieved a sensitivity of 93.2% and a specificity of 100%. Furthermore, this kit was able to detect SARS-CoV-2 in some patients in the early stages of the illness (within 1 week) with undetectable levels of viral RNA, and combination with the molecular test was able to raise the sensitivity for detecting COVID-19 (p<0.001).5
In addition to the protein reagents, due to the global impact of COVID-19, these tests need to be able to be scaled quickly, deliver high-throughput analysis, and be distributed worldwide. In April, scientists at the Policlinico San Matteo Hospital in Italy developed a fully automated test that was able to perform up to 170 samples per hour. The chemiluminescent assay detected SARS-CoV-2 IgG antibodies against the S1 and S2 domain of the S protein and was highly specific.10
ACROBiosystems Offer High-Quality Antigens and Antibodies
ACROBiosystems is a leading manufacturer of recombinant proteins and has developed over a hundred products to support the researches against SARS-CoV-2, including a serial of high-quality antigens and antibodies for the development of SARS-CoV-2 serological diagnostic tests. A summary of these products is shown in Table 1.9
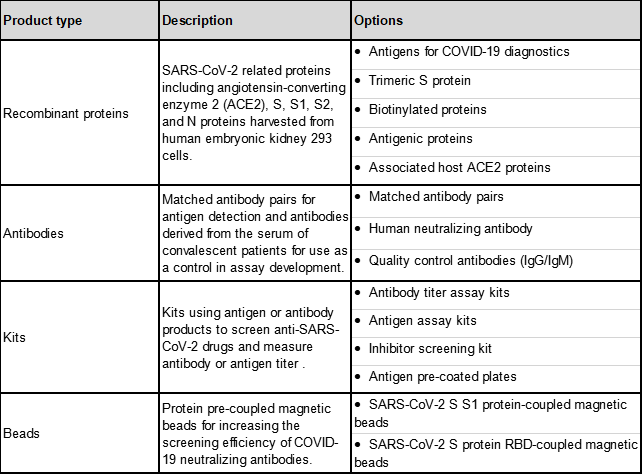
Table 2. SARS-CoV-2 products from ACROBiosystems.9
Hot Products
Matched antibody pairs for antigen detection:
· High sensitivity of 12 pg/mL (ELISA)
· Super affinity of 10pM level (BLI)
· High specificity: no cross-reactivity with other coronaviruses
· High batch-to-batch consistency & bulk order available
· Suitable for different antigen detection methods, such as CLIA, ELISA, GICA, etc.
Antigens for antibody detection:
· Customized formulation and bulk order available
· High purity and batch-to-batch consistency
· Validated functionality
These products are scalable and can be distributed globally.9 For more information, please visit https://www.acrobiosystems.com/A1126-Antigens--Antibodies-for-Diagnostic-Kit-Development.html.
This web search service is supported by Google Inc.






