 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> CMC Production Process of Adoptive NK Cell Therapies
![]() Recommended CMC production process of other cell therapies for you
Recommended CMC production process of other cell therapies for you
CMC Production Process of Adoptive NK Cell Therapies
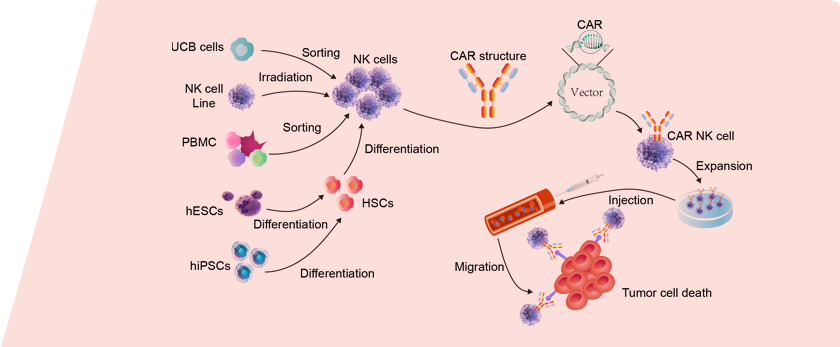

Activation and expansion of NK cells from different sources

Enhancement of NK cell activity
Activation and expansion of NK cells from different sources
![]() NK cell activation products
NK cell activation products
 Anti-4-1BB AntibodyComing Soon
Anti-4-1BB AntibodyComing Soon
iPSC/ESC Source
PBMC Source
UCB Source
NK Cell Lines Source
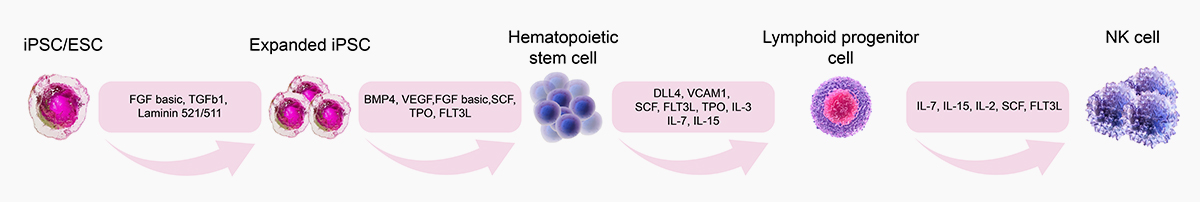
![]() GMP Grade DLL4, VCAM1:Eliminate the need of feeder cells in culture systems and support differentiation of iPSCs to NK cells
GMP Grade DLL4, VCAM1:Eliminate the need of feeder cells in culture systems and support differentiation of iPSCs to NK cells
![]() Validated activity by iPSC/ESC to NK cell differentiation
Validated activity by iPSC/ESC to NK cell differentiation
![]() Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
![]() Comprehensive quality release verification, with 16 quality control indicators
Comprehensive quality release verification, with 16 quality control indicators
![]() Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
![]() Produced in a Pharmaceutical-grade production facility
Produced in a Pharmaceutical-grade production facility
![]() Support for online and offline audits
Support for online and offline audits
![]() Completion of FDA Drug Master File (DMF) registration
Completion of FDA Drug Master File (DMF) registration
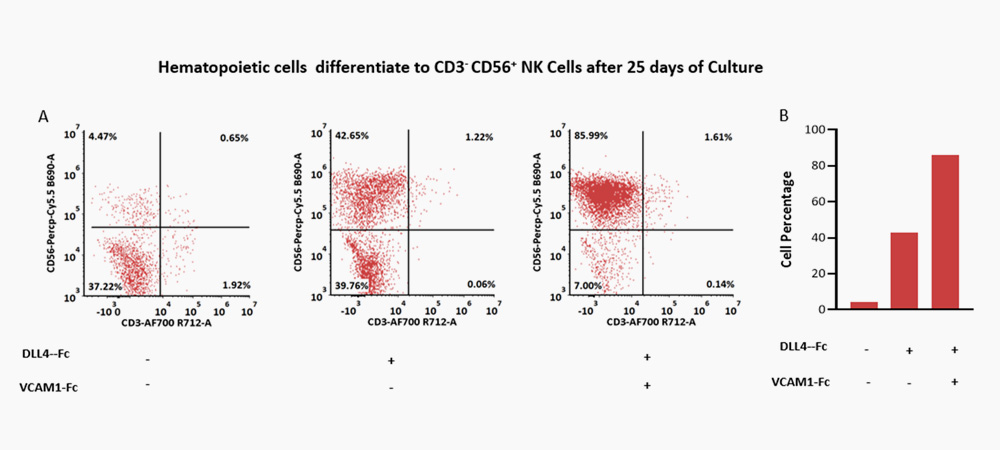
The combination of DLL4 (GMP-DL4H28) & VCAM1 (GMP-VC1H25) could significantly facilitate the differentiation efficiency of CD56+ CD3- NK cells.
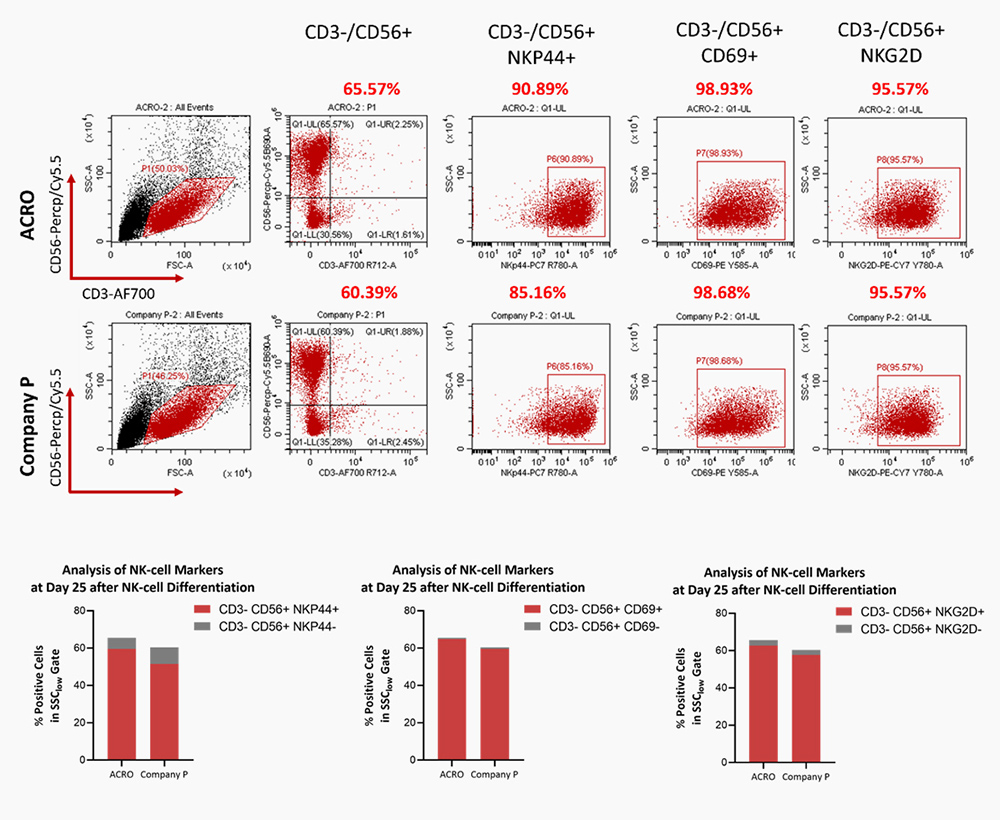
SCF(GMP-SCFH25), Flt3L(GMP-FLLH28), IL-7(GMP-L07H24) could significantly promote the HSPC differentiation to NK cells, comparable to Company P.
Recommended cytokines combination:IL-2+IL-15 IL-15+IL-21 IL-2+IL-18
![]() Validated activity by PBMC to NK cell differentiation
Validated activity by PBMC to NK cell differentiation
![]() Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
![]() Comprehensive quality release verification, with 16 quality control indicators
Comprehensive quality release verification, with 16 quality control indicators
![]() Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
![]() Produced in a Pharmaceutical-grade production facility
Produced in a Pharmaceutical-grade production facility
![]() Support for online and offline audits
Support for online and offline audits
![]() Completion of FDA Drug Master File (DMF) registration
Completion of FDA Drug Master File (DMF) registration
Human PBMCs were cultured with IL-2 (Cat. No. GMP-L02H14), IL-15 (Cat. No. GMP-L15H13), IL-18 (Cat. No. IL8-H5114), IL-21 (Cat. No. GMP-L21H25). The result shows that they are functional and can promote the expansion of these cells with a reasonable cell viability.
Human PBMCs were cultured with IL-2 (Cat. No. GMP-L02H14), IL-15 (Cat. No. GMP-L15H13), IL-18 (Cat. No. IL8-H5114), IL-21 (Cat. No. GMP-L21H25). The result shows that they are functional and can increase the percentage of the CD3-CD56+ cells.
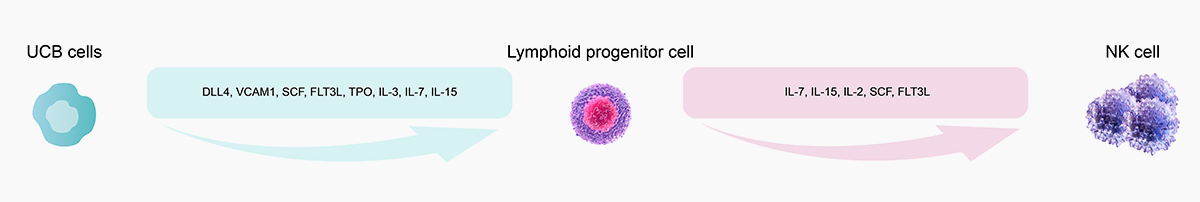
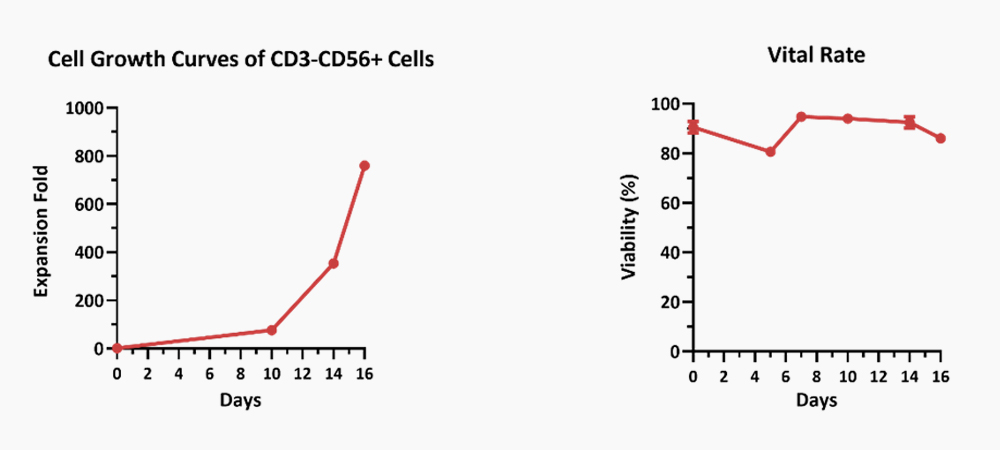
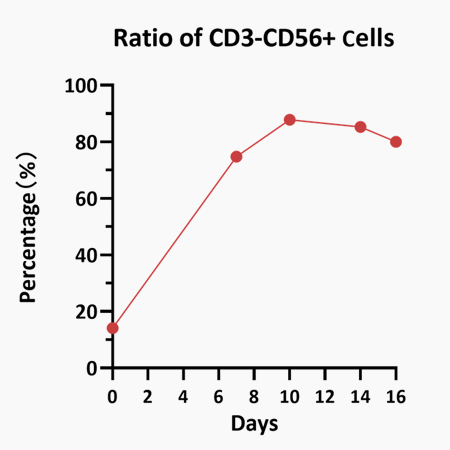
![]() Validated activity by UCB to NK cell differentiation
Validated activity by UCB to NK cell differentiation
![]() Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
![]() Comprehensive quality release verification, with 16 quality control indicators
Comprehensive quality release verification, with 16 quality control indicators
![]() Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
![]() Pharmaceutical-grade production facility
Pharmaceutical-grade production facility
![]() Support for online and offline audits
Support for online and offline audits
![]() Completion of FDA Drug Master File (DMF) registration
Completion of FDA Drug Master File (DMF) registration
![]() Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
Production and quality control are carried out under strict GMP systems and comply with regulations from multiple countries
![]() Comprehensive quality release verification, with 16 quality control indicators
Comprehensive quality release verification, with 16 quality control indicators
![]() Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
Enhanced safety (sterile, no mycoplasma, no exogenous virus, animal free production system, and various impurities residual detection)
![]() Pharmaceutical-grade production facility
Pharmaceutical-grade production facility
![]() Support for online and offline audits
Support for online and offline audits
![]() Completion of FDA Drug Master File (DMF) registration
Completion of FDA Drug Master File (DMF) registration
Enhancement of NK cell activity
![]() High purity, high enzyme activity, high cleavage efficiency
High purity, high enzyme activity, high cleavage efficiency
![]() Possesses nuclear localization signals to enhance editing efficiency
Possesses nuclear localization signals to enhance editing efficiency
![]() Aseptic, ultra-low endotoxin
Aseptic, ultra-low endotoxin
![]() Produced in GMP-compliant facilities and undergoes QC testing
Produced in GMP-compliant facilities and undergoes QC testing
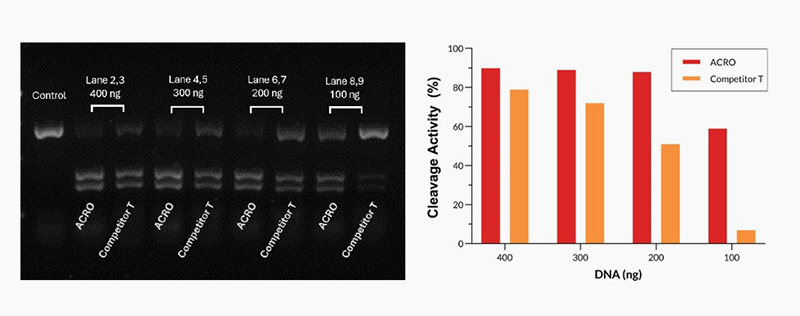
Different amounts of Cas9 were incubated with the same amount of excess gRNA and plasmid for 60 minutes at 37°C. When using 400-200 ng Acro Cas9, the cutting efficiency is greater than 90%. In comparison, when using a 200 ng Competitor T, the cutting efficiency is only about 50%.

Resources
Cytokines residue ELISA kits
IL-2, IL-4, IL-6, IL-7, IL-10, IL-15, IL-21, IL-1B, TNF-alpha, GM-CSF residue kits.
HCD detection
Enzyme residue kit
DNase Activity Assay Kit (Fluorescence)
Mono-growth factor detection
Multiplex detection
This web search service is supported by Google Inc.