





























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> CelThera™ GMP T Cell Expansion Medium
Chemically Defined AOF No Serum or SR Needed Fast Cell Expansion

T lymphocytes play a crucial role in the human immune system, and utilizing the inherent functions of specific T cells to combat diseases has become a breakthrough technological approach. T cell culture medium provides the nutritional matrix required for T cell proliferation. By simulating the in vivo microenvironment, isolated T cells can expand effectively in vitro while maintaining their structure and function. The development of T cell culture media has progressed through three important stages, among these, chemically defined media have become the preferred choice, as they ensure batch-to-batch consistency, traceability, and product stability, becoming the mainstream choice in the future.
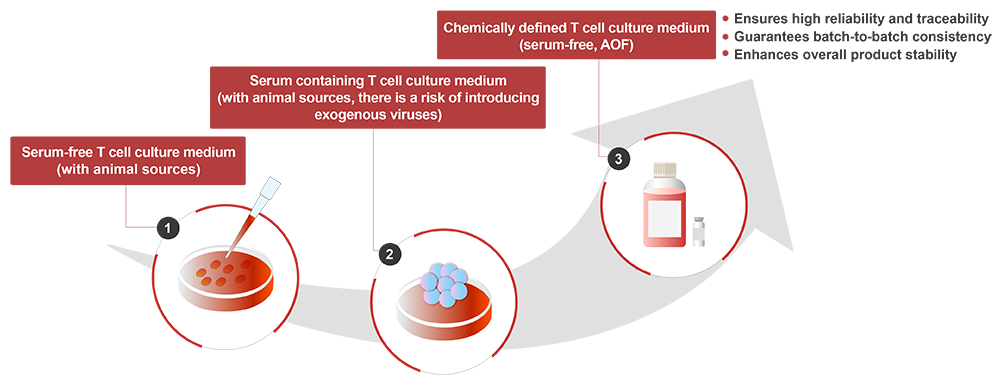
ACROBiosystems’ newly launched CelThera™ GMP T Cell Expansion Medium used an innovative chemical limited formula, without serum and animal derived component, focusing on improving the stability and controllability of T cell culture, helping to enhance T cell proliferation and vitality, and improving overall production efficiency.
Chemically defined, animal-origin free (AOF) and high batch-to-batch consistency.
No additional serum or serum replacement required to maintain optimal expansion rates.
Enables high fold expansion of T cells in a reduced timeframe (100-fold expansion in < 7 days, 3,000-fold expansion in 13 days).
Achieves a balanced CD4+: CD8+ 1:1 T-cell ratio more quickly.
Faster promotion of TN/SCM ratio increase in cells.

Promote rapid expansion of T cells, CAR-T cells, TCR-T cells, peripheral blood lymphocytes (PBL) and other T source cells.
Support scaling up from preclinical development to production.
Produced under GMP conditions.
Provide complete declaration support documents, complete set of methodological validation reports and other documents, FDA DMF filed.
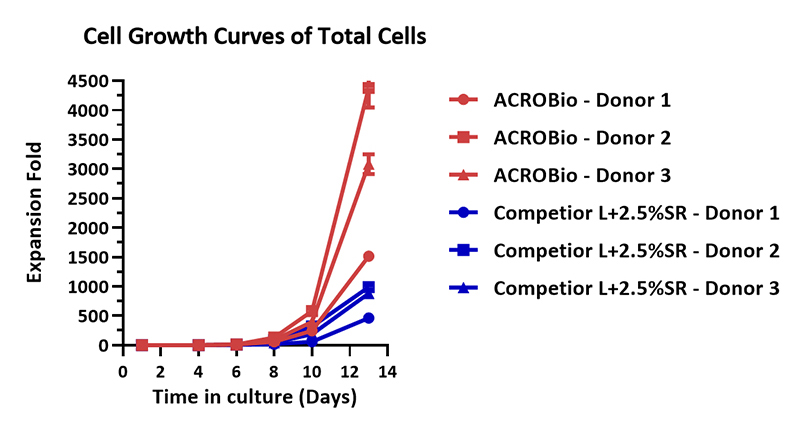
Human PBMCs were cultured with GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) with CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101) or T cell culture medium (Competitor L +2.5% SR) for two weeks. The result shows that CelThera™ GMP T Cell Expansion Medium (ACROBiosystems) can be comparable with the Competitor L +2.5% SR. Notably, the cells exhibits better expansion in CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101).
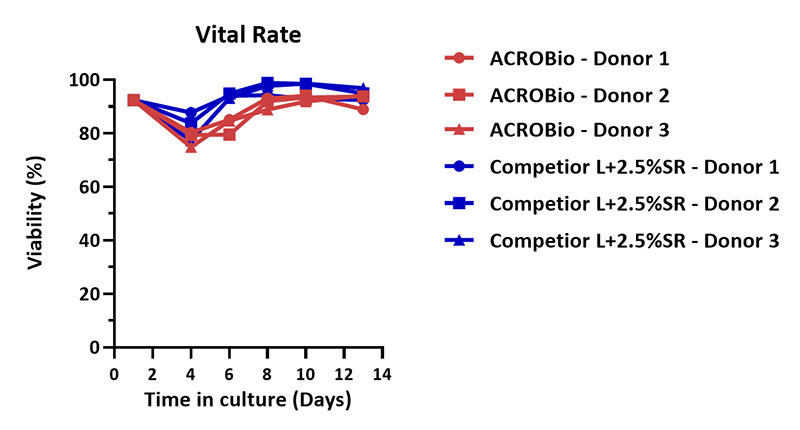
Human PBMCs were cultured with CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101) or T cell culture medium (Competitor L +2.5% SR) for two weeks. The result shows that CelThera™ GMP T Cell Expansion Medium (ACROBiosystems) can be comparable with the Competitor L +2.5% SR.
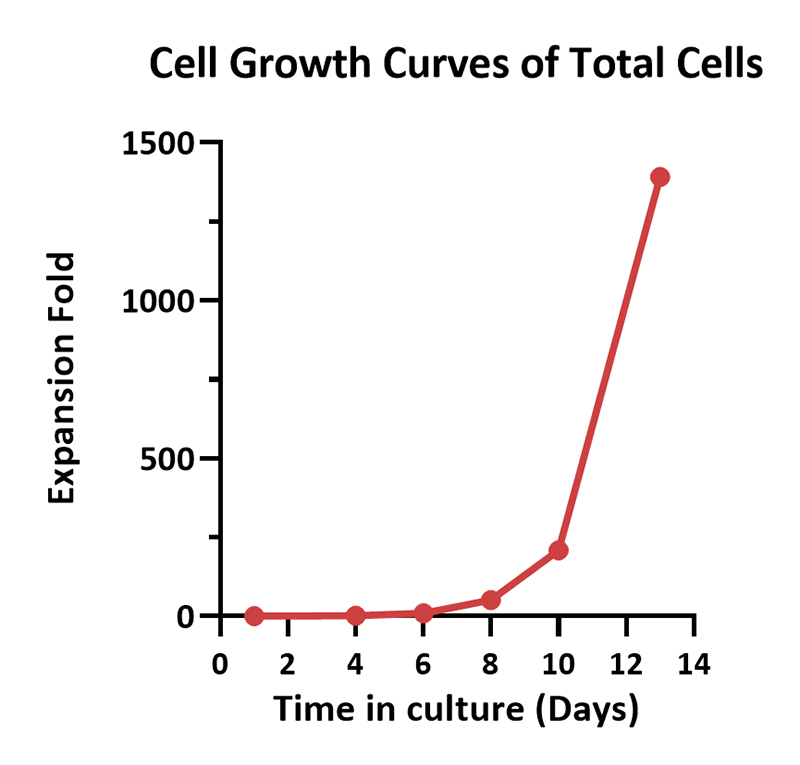
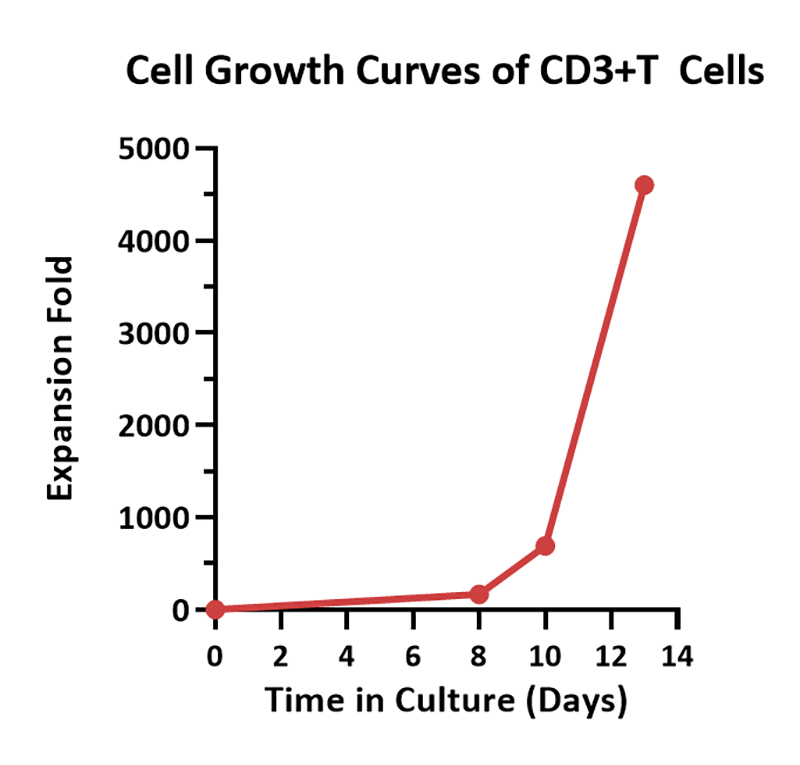
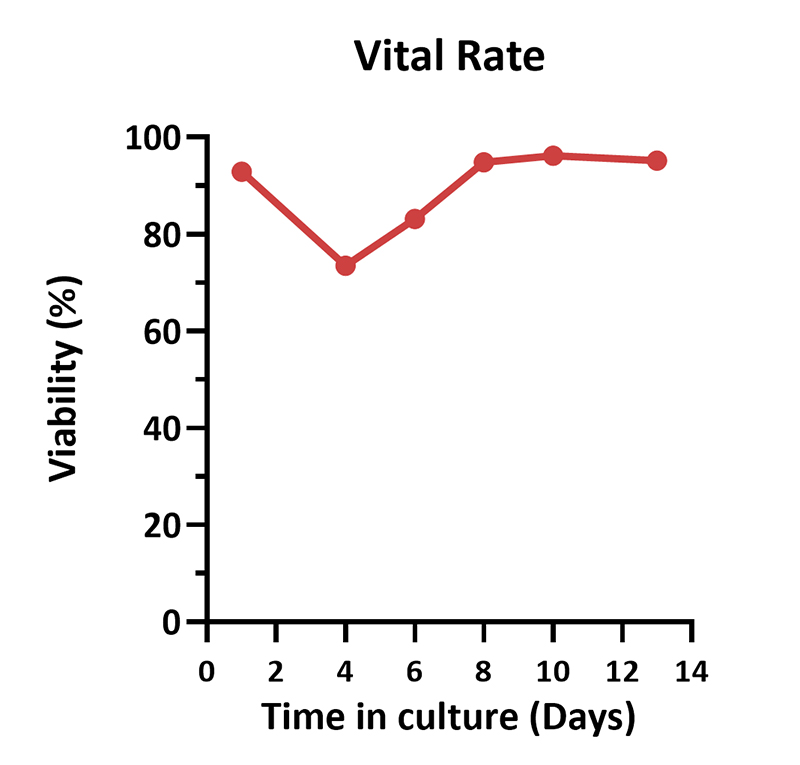
Human PBMCs were cultured with GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) with CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101 & GMP-CM3101-1) for two weeks. The result shows that CelThera™ GMP T Cell Expansion Medium with GMP Human IL-2 Protein can promote the expansion of these cells with a reasonable cell viability.
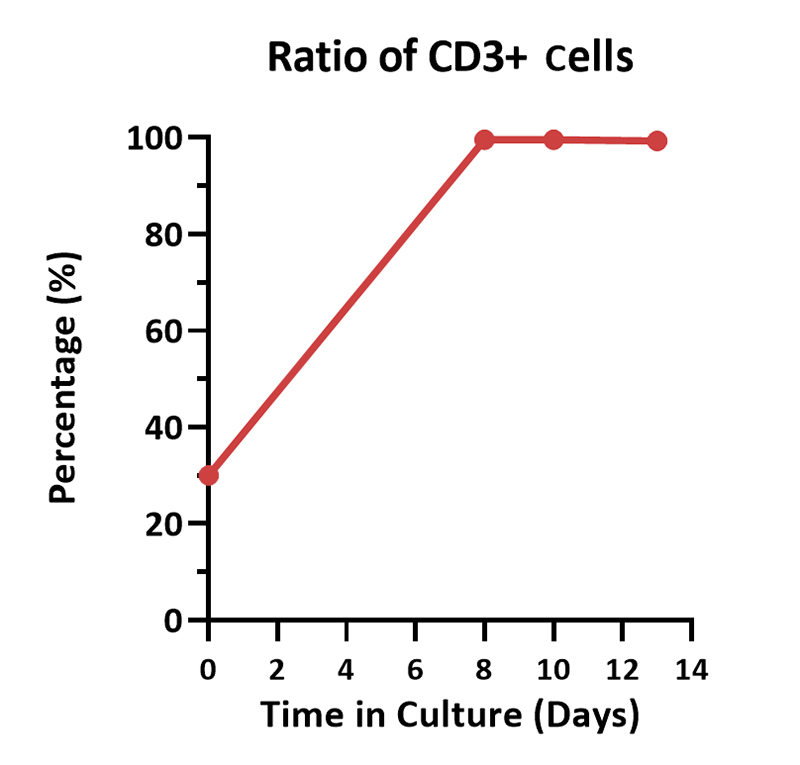
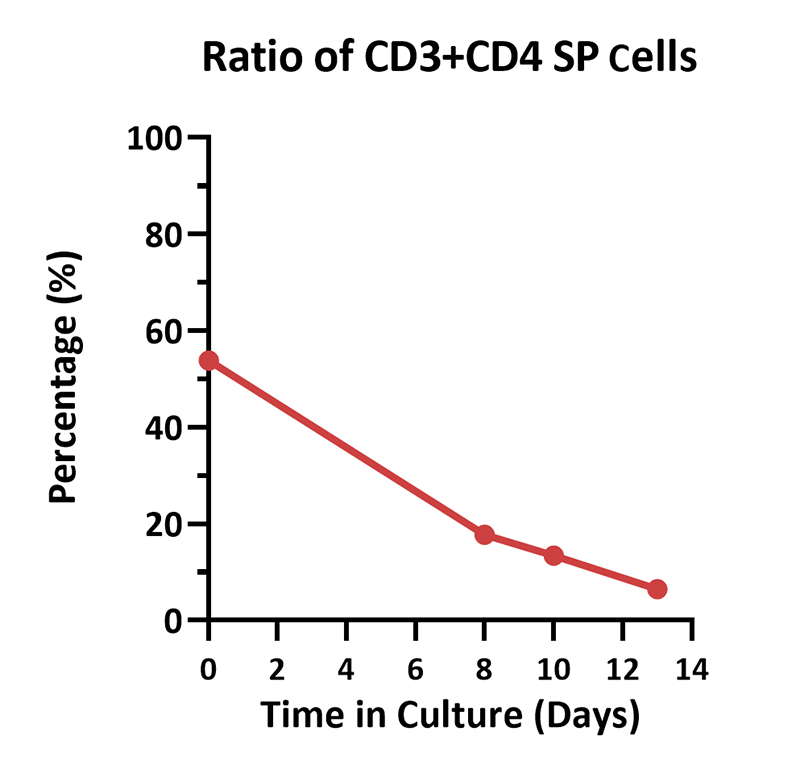
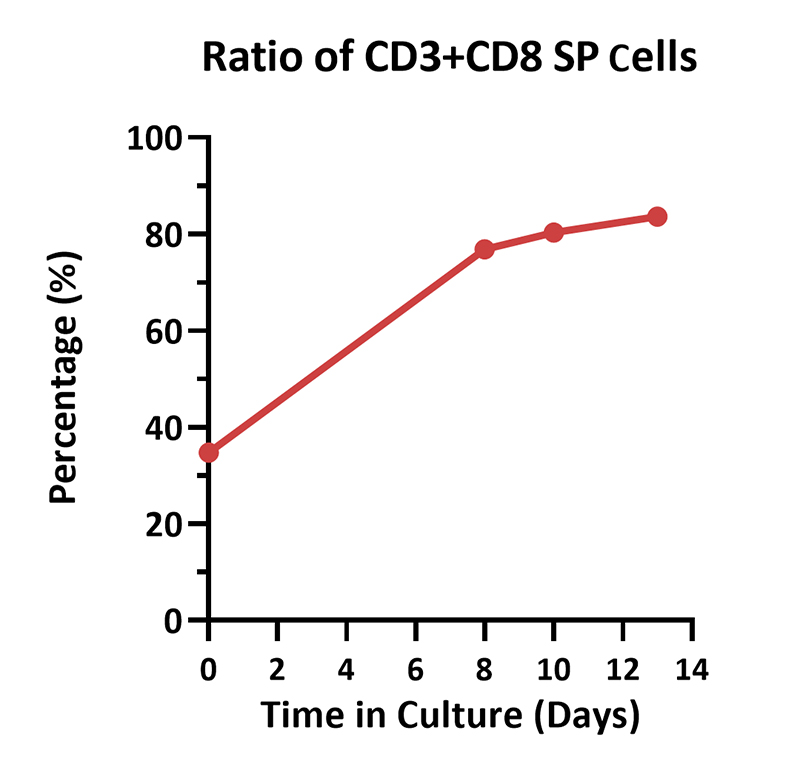
Human PBMCs were cultured with GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) with CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3101 & GMP-CM3101-1) for two weeks. The result shows that CelThera™ GMP T Cell Expansion Medium with GMP Human IL-2 Protein can increase the percentage of the CD3+ cells. With the post-culture time, the proportion of CD4 cells gradually decreased, and the proportion of CD8 cells gradually increased.
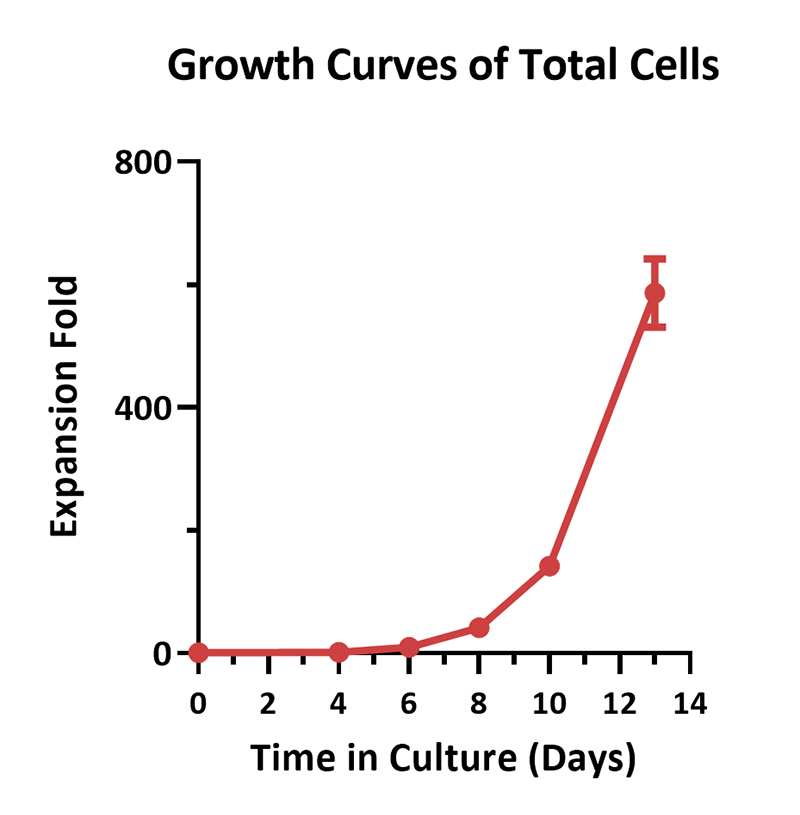
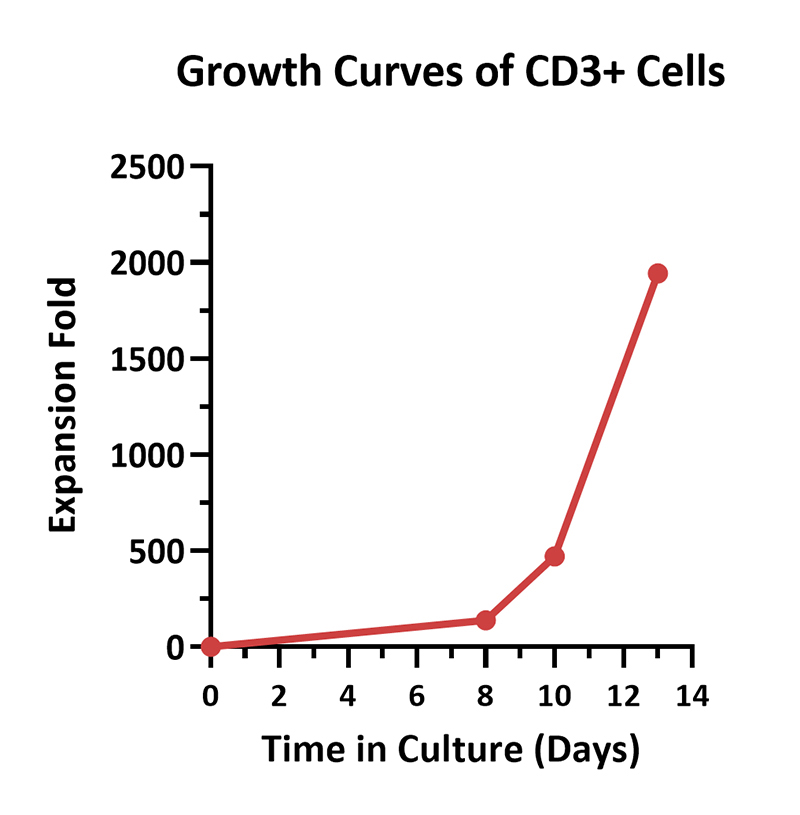
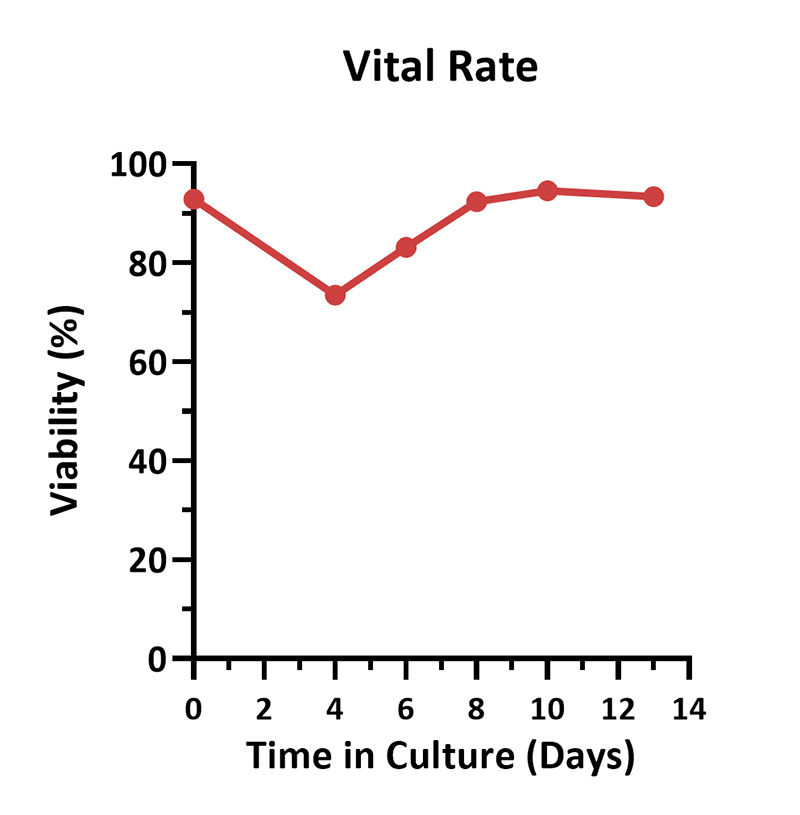
Human PBMCs were activated using 0.2 µg/mL GMP Monoclonal Anti-Human CD3 Antibody (OKT3) (ACROBiosystems, Cat. No. GMP-MC0323) and 1 µg/mL GMP Monoclonal Anti-Human CD28 Antibody (ACROBiosystems, Cat. No. GMP-MC2824), cultured with CelThera™ GMP T Cell Expansion culture medium (ACROBiosystems, Cat. No. GMP-CM3101) supplemented with 500 IU/mL GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) for two weeks. The results showed that GMP human IL-2 protein, GMP monoclonal anti-human CD3 antibody (OKT3), GMP monoclonal anti-human CD28 antibody and CelThrea™ GMP T cell expansion medium could be used to culture T cells in a 3L large system. It can efficiently expand cells with high viability.
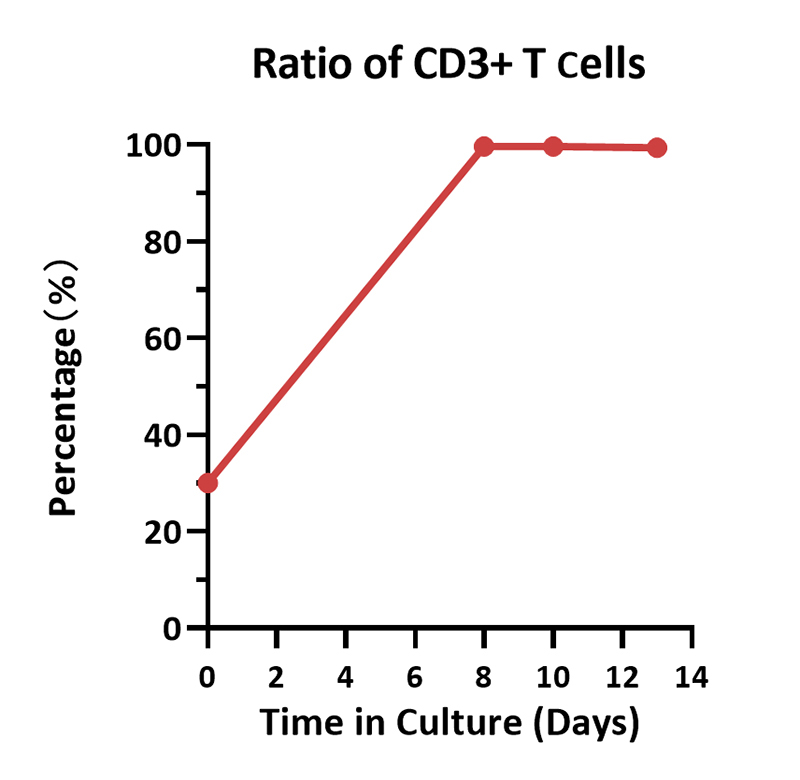
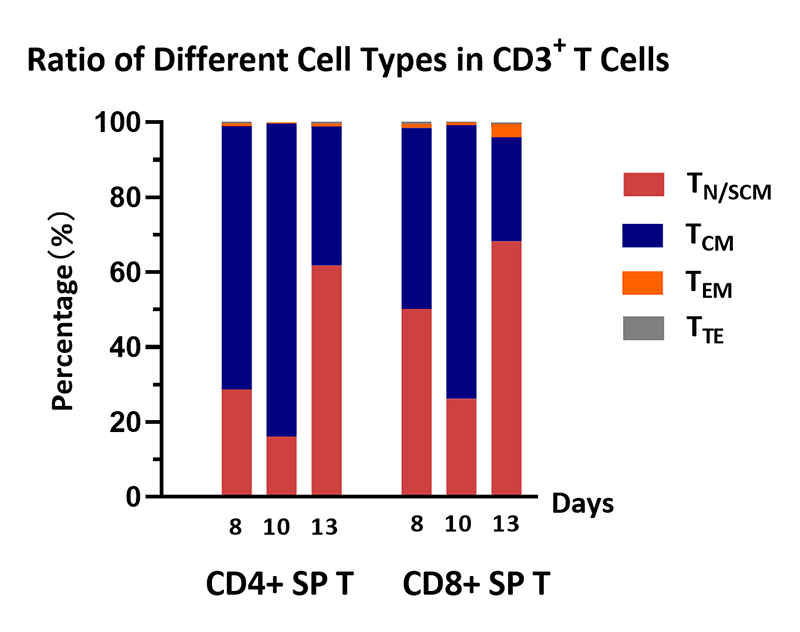
Cell ratio of the human various T cells. Human PBMCs were activated using 0.2 µg/mL GMP Monoclonal Anti-Human CD3 Antibody (OKT3) (ACROBiosystems, Cat. No. GMP-MC0323) and 1 µg/mL GMP Monoclonal Anti-Human CD28 Antibody (ACROBiosystems, Cat. No. GMP-MC2824), and cultured in CelThera™ GMP T Cell Expansion culture medium (ACROBiosystems, Cat. No. GMP-CM3101) supplemented with 500 IU/mL GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) for two weeks. The result shows that percentage of CD3+ cells in living cells is above 99%. At the end of the culture process, the majority memory phenotype of T cells are TN/SCM, followed by TCM, which means that the cell harvest will have a promising effect, such as sustained proliferation, prolonged survival, and so on.
GMP-CM3101-1 is our GMP T Cell Expansion Medium supplement, formulated without serum.
The base culture medium does not contain any growth factors or antibody components. These should be added separately, according to your experimental requirements.
After mixing two components, they can be stored at 4 ℃ for 1 month, but it is recommended to use them up within 2 weeks as much as possible after mixing.
To use GMP-CM310-1, thaw it at room temperature. Once thawed, transfer the entire contents of the GMP-CM310-1 vail into the GMP-CM3101 base medium. Mix thoroughly before use.
This web search service is supported by Google Inc.







