





























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Insights > Variant-Specific Antibody for Omicron-Based and Polyvalent Vaccine R&D There have been more than 10 billion doses of vaccines administered globally for protecting people from COVID-19. However, in the three months since the Omicron variant (B.1.1.529) emerged, its toll across the globe has been devastating. In the United States, both the average new cases and daily deaths due to Omicron have already exceeded those during the delta variant’s peak last September. The repeated outbreaks and the continued spread of the new variant have given rise to people’s thinking for the improvement of existing and the development of future vaccines.
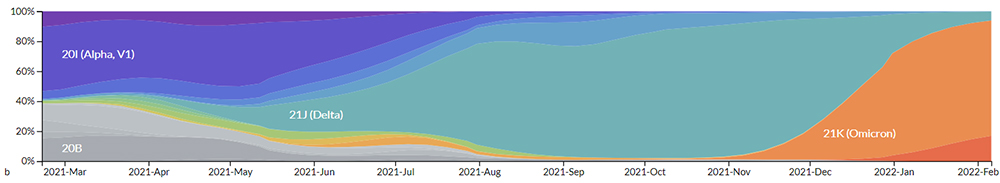
(Genomic epidemiology of novel coronavirus - Global subsampling)
Spike protein plays an essential role in the COVID-19 infection pathway, in which it binds to human cell surface receptors to trigger cell fusion and invasion. Previous studies have shown that mutations in spike proteins can enhance viral replication and immune escape, making the virus more resistant to vaccines. Omicron mutant has more than 30 mutations only in spike protein, including some important mutation sites of all four VOC simultaneously, which greatly enhances its immune escape ability.

Related article: According to the mutations: Why is Omicron causing such concern?
According to the literature, the titer of neutralizing antibody of vaccinated people's serum is significantly reduced against Omicron. The breakthrough infection can occur even in people who have completed two doses of vaccines. Roy Chemaly, the Infection and disease control specialist from MD Anderson USA, said that the number of breakthrough infection cases due to Omicron has far exceeded that of Delta. Therefore, developing new vaccines with sufficient protection against Omicron mutant has become an urgent need.

(Neutralizing titers of sera from BNT162b2 vaccinated recipients)
As the Omicron variant of SARS-CoV-2 continues its global rampage, vaccine producers are pouring resources into the vaccine development shots tailored to the highly transmissible variant. Pfizer Inc. and BioNTech are developing the Omicron-based vaccine and will launch as early as March. Moderna, a U.S. vaccine company, is also developing a new generation of COVID-19 vaccine, mrna-1273.529 (also known as mRNA-Omicron), as a booster shot and is now in clinical trials. In China, the team of Microbiology of the University of Hong Kong, as the first research team in Asia to successfully isolate and cultivate the Omicron mutant strain, announced the isolated strain have been offered to the Chinese Center for Disease Control and Prevention, Sinovac biology and Sinophem for research and vaccine development on December 9 and December 10 respectively. Consino Bio also announced their plan for developing a COVID-19 vaccine against the Omicron variant last year.
However, a raft of early animal studies suggest that Omicron-specific boosters of Moderna offer no advantage over a third dose of current vaccines, which suggest that the development and improvement for vaccines needs to continue.
Considering the continuesly mutation of the SARS-CoV-2 various and the rapid transmission, polyvalent vaccine is expected to be the most effective defense in the war against COVID-19. The development of vaccine has strict requirements for its immunogenicity and effectiveness; therefore, the detection of antigen content is an indispensable step in the process of vaccine development. For the R&D of polyvalent vaccine, it is necessary to establish a method for quantitative detection of mutated antigen.
To meet the mutant antigen quantitative detection requirement during the process of polyvalent vaccine R&D, ACROBiosystems immediately developed an Omicron-specific antibody (Cat.No. SPD-M305). The antibody can only bind to Omicron Spike RBD and not bind to other SARS-CoV-2 strains.
Main features:
- High bioactivity: the affinity constant of Omicron-specific antibody and Omicron Spike RBD is 9.07 nM in BLI assay;
- High purity: the purity of this antibody is more than 95% verified by SDS-PAGE;
- Can be used for mutant antigen quantitative detection requirement during the process of polyvalent and Omicron-based vaccine R&D.
Assay Data
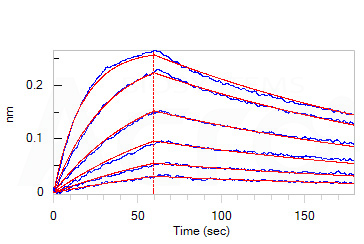
Loaded Monoclonal Anti-SARS-CoV-2 Spike RBD Antibody, Mouse IgG1 (Cat. No. SPD-M305) on AMC Biosensor, can bind SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (Cat. No. SPD-C522e) with an affinity constant of 9.07 nM as determined in BLI assay (ForteBio Octet Red96e).

Immobilized SARS-CoV-2 Spike RBD (Omicron, Cat.No. SPD-C522e) can bind Anti-SARS-CoV-2 Spike RBD Antibody, Mouse IgG1(Cat.No.SPD-M305) with a linear range of 0.4-12.5 ng/mL. The antibody does not bind Spike RBD of WT (Cat.No. SPD-C52H1), Alpha (Cat.No. SPD-C52Hn), Beta (Cat.No. SPD-C52Hp), Delta(Cat.No. SPD-C52Hh) and Gamma (Cat.No. SPD-C52Hr).
This web search service is supported by Google Inc.






