 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Insights > Multiple Sclerosis: Etiology, Pathology, Pathogenesis and Therapeutic Drugs Multiple sclerosis(MS)is an immune-mediated inflammatory demyelinating disease of the central nervous system. A relapsing-remitting course may characterize the clinical course of MS, a progressive disease course, or a combination thereof. There are approximately 2 million MS patients worldwide, and it is the most common cause of permanent disability in young adults other than traumatic brain injury, resulting in a large socioeconomic burden. Approximately 1 million people with MS in the United States alone, and the associated costs exceed $24 billion.
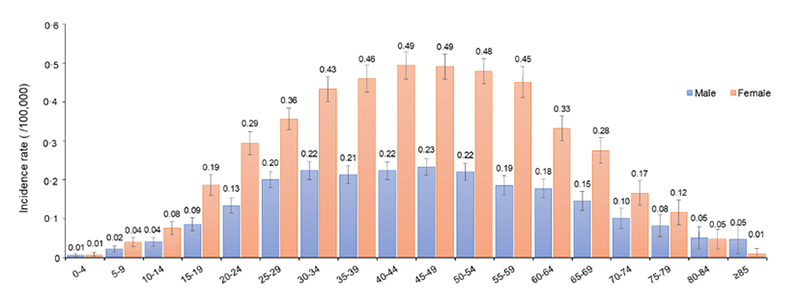
Prevalence of MS in different age groups
The pathological damage in MS includes inflammatory demyelination, gliosis, and sclerotic plaque formation. In recent years, it has been shown that MS can also cause neuronal degeneration and primary axonal damage, which manifests as damage to the brain's gray matter, especially the cerebral deep gray matter. Its etiology and pathogenesis are complex, and numerous clinical and experimental studies have shown that the occurrence of MS is the result of the interaction of genetic and environmental factors:
- Genetic factors including both ethnicity and individual susceptibility;
- Environmental factors include viral infections, environmental pollution, frequent immunizations, genetically modified foods, and various surgical traumas, among many others.
Experimental virological and immunological studies have revealed that when a virus invades the body, mononuclear macrophages in the body phagocytose and digest the virus and transmit the viral antigen signal, which shares antigenicity with myelin basic protein, to helper T cells, which activate into the CNS, activate effector T cells, release a large number of cytokines, and activate complement and B cells, leading to oligodendrocyte degeneration and myelin damage—ultimately leading to MS pathogenesis. This hypothesis has been confirmed by numerous studies on animal models of experimental autoimmune encephalomyelitis (EAE) and is now increasingly accepted as molecular mimicry.
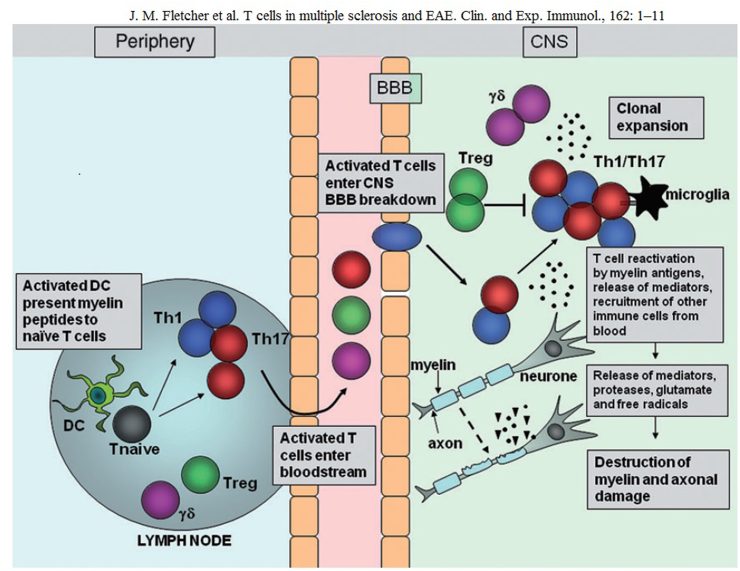
T-cell migration in the central nervous system during experimental autoimmune encephalomyelitis (EAE)
There are currently more than 20 drugs approved for the treatment of MS worldwide, mainly divided into small chemical molecules, interferons, and monoclonal antibodies. Glucocorticoids were the mainstay of early MS treatment, but they were ineffective in reducing the number of relapses and the rate of disease progression; in the 1990s, interferon was used in the clinical treatment of the disease; in 1996, the heavyweight product glatiramer acetate was marketed; since this century, several oral small-molecule chemical drugs and monoclonal antibodies have been introduced, providing multiple avenues for the treatment of MS.
>> The integrin alpha-4 (ITGA4) gene is thought to be one of the genetic factors affecting MS pathogenesis, and its encoding of ITGA4 assists in the migration of leukocytes across the blood-brain barrier in MS. Therefore, ITGA4 is a potent therapeutic target for MS. Natalizumab, developed for this target, was approved by the US FDA in 2004 to treat MS and Crohn's disease. Natalizumab effectively treats symptoms of both diseases, increases remission rates and, prevents relapses, vision loss, cognitive decline, and significantly improves the quality of life in MS patients.
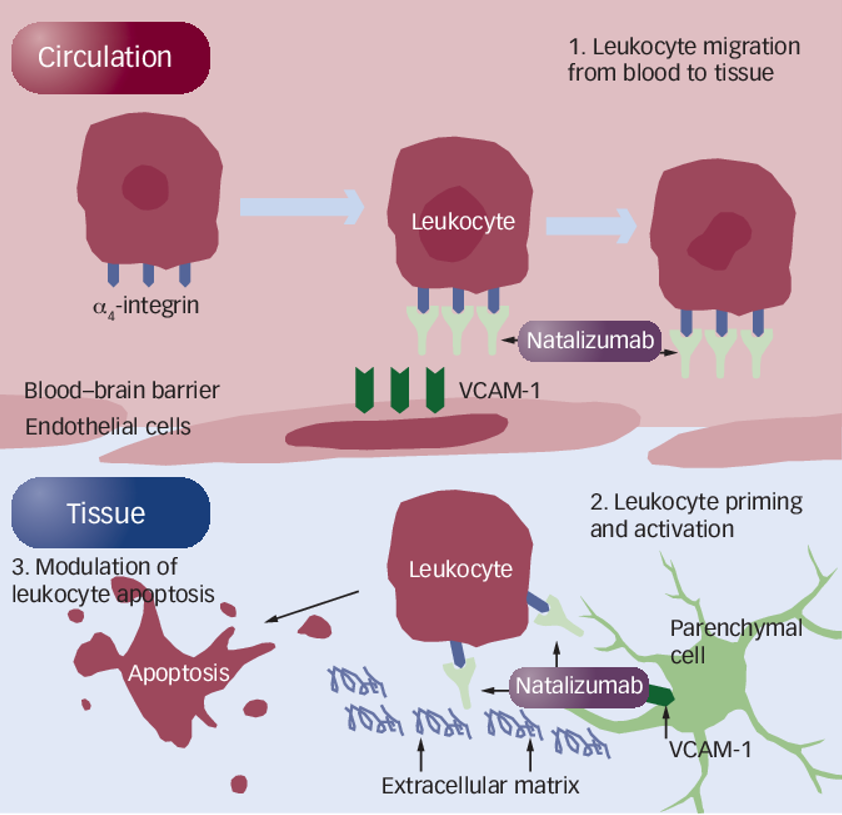
Mechanism of action of natalizumab in the treatment of MS
>> The interferon-alpha/beta receptor alpha chain(IFNAR1) gene encodes a type I membrane protein that constitutes one of the two chains of interferon α and β receptors. The receptor for type I interferon consists of two subunits, IFNAR1 and IFNAR2. Binding of type I IFN by IFNAR1 activates the JAK-STAT signaling pathway, which is essential for regulating growth, survival, differentiation, pathogen resistance, and antiviral immunity, triggering many protein IFN/IFNAR1 activation of immune mechanisms is also an important target for MS therapy.
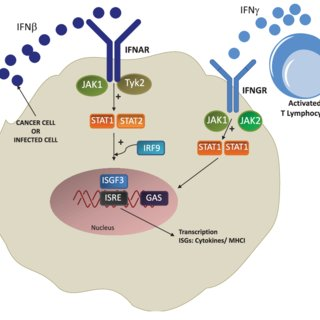
Signal pathway of IFN/IFNAR1
>> Sphingosine-1-phosphate receptor 5 (S1PR5) is predominantly expressed in CNS white matter bundles and significantly expressed by oligodendrocytes and is involved in and regulates natural killer cell trafficking. Modulators targeting S1PR are a relatively new class of therapies, being the first oral therapy for MS, the first approved for pediatric MS, and the first to prove effective in secondary progressive MS (SPMS). Its primary mechanism of action is through binding to S1PR isoforms on lymphocytes, leading to receptor internalization and loss of responsiveness to the S1P gradient that drives lymphocyte drainage from lymph nodes. The reduction in circulating lymphocytes may limit the migration of inflammatory cells to the CNS. Four S1PR modulators (fingolimod, Siponimod, ozanimod, ponesimod) have been approved to treat MS.
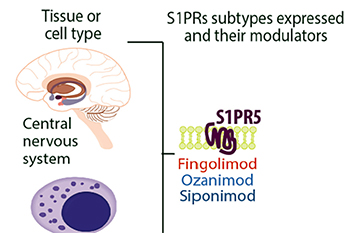
S1PR5 expression in the CNS system
>> Ocrelizumab, a monoclonal antibody targeting CD20, was approved for marketing in March 2017 as the first drug for primary progressive MS. Recent clinical studies have shown that the use of Rituximab in patients with relapsing-remitting MS resulted in a rapid reduction of B cells, as well as myelin lesions and clinical relapses, with effects lasting 3-12 months. In a phase II study of Rituximab in patients with relapsing-remitting MS, significant reductions in MRI and clinical markers of disease activity were shown. 104 patients with RRMS were randomly assigned to 2 x 1000 mg of intravenous Rituximab or placebo and monitored for 48 weeks. The Rituximab group showed a significant reduction in the number of contrast-enhanced MRI lesions (p < 0.001) and T2 lesion volume (p = 0.04) at weeks 24 and 36, respectively, compared with the placebo group. The Rituximab group further reduced the annualized MS recurrence rate, statistically significant at week 24 but not at week 48.
>> CD52 is a cell surface antigen present on all lymphocytes and monocytes. Alemtuzumab, a humanized monoclonal antibody targeting CD52, was approved for marketing by the FDA in May 2001 for leukemia treatment and was granted MS orphan drug status by Mexico in 2014. Two-phase III clinical studies confirmed the effectiveness of alemtuzumab, with a 55% lower annual relapse rate and similar disability progression rates compared to interferon. However, because alemtuzumab has serious adverse effects, particularly secondary autoimmune disease, in 2019, the European Medicines Agency recommended that alemtuzumab should be restricted for use in adults with MS.
MS is a relatively common immune-mediated disorder of the central nervous system that can cause severe disability and reduce the quality of life. Following the understanding of this disease's pathogenesis and disease process, monoclonal antibodies targeting a range of immune pathways have made compelling advances in MS treatment outcomes. The development of highly effective therapies has led to near-complete control of relapsing disease and focal brain inflammation. However, the effectiveness of disease-progressing therapeutics remains inadequate, as current treatments provide only partial protection against neurodegenerative lesions in MS. We should seek an evidence-based, personalized approach to MS treatment and management that advances early treatment. Finally, the field is developing new methods to identify and assess the remyelination capacity and neuroprotective potential of drugs, which will advance ongoing advances in MS therapeutics.
Aneuro focuses on brain science research and development of MS drugs and therapeutics and can provide integrin alpha-4 (ITGA4), Interferon-alpha/beta receptor alpha chain(IFNAR1),CD20, CD52 and other target proteins, and more MS diagnosis and treatment-related proteins are under development (including S1PR5).
>>>Learn more about Aneuro: advancing neuroscience research
ACRO is developing more proteins for AD diagnosis and treatment. If you have any needs or more development suggestions, don't hesitate to contact us.
1. Tian DC, Zhang C, Yuan M, Yang X, Gu H, Li Z, Wang Y, Shi FD. Incidence of multiple sclerosis in China: A nationwide hospital-based study. Lancet Reg Health West Pac. 2020 Aug 6;1:100010. doi: 10.1016/j.lanwpc.2020.100010.
2. Cavallo S. Immune-mediated genesis of multiple sclerosis. J Transl Autoimmun. 2020 Jan 28;3:100039. doi: 10.1016/j.jtauto.2020.100039.
3. Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011 Oct;164(4):1079-106. doi: 10.1111/j.1476-5381.2011.01302.x.
4. Bates, David W. "Natalizumab (Tysabri ®) – Redefining Efficacy in Multiple Sclerosis – Data from Clinical Trials to Postmarketing Experience." (2010)
5. Gonzalez-Cao M, Karachaliou N, Santarpia M, Viteri S, Meyerhans A, Rosell R. Activation of viral defense signaling in cancer. Ther Adv Med Oncol. 2018 Aug 29;10:1758835918793105. doi: 10.1177/1758835918793105.
6. Mark B. Skeen, MD. Sphingosine-1-Phosphate Modulators for Multiple Sclerosis. https://practicalneurology.com/articles/2020-feb/sphingosine-1-phosphate-modulators-for-multiple-sclerosis
This web search service is supported by Google Inc.