 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Insights > The Hundred-Billion-Level Weight Loss Market: Are Peptide Drugs the Only Option? 
In 2023, the global anti-obesity drug market reached $6 billion, and Goldman Sachs forecasts a more than 16-fold increase to reach 100 billion by 2030.
In recent years, glucagon-like peptide-1 receptor (GLP-1R) has become a key target in diabetes and obesity treatment due to its role in regulating blood glucose and weight. Peptide-based GLP-1R agonists have shown significant efficacy in lowering blood glucose and promoting weight loss. Advances in antibody drug technology have led to the emergence of GLP-1R antibody drugs, offering potential for longer-lasting, more effective, and safer treatments.
GLP-1R, a key member of the class B G protein-coupled receptor (GPCR) family, features a characteristic seven-transmembrane domain (TMD) and an extracellular domain (ECD) of 120-160 amino acids. Both the ECD and TMD are essential for GLP-1R's interaction with its peptide ligand GLP-1 and subsequent activation. The ECD binds the ligand, anchoring it to the transmembrane region, which induces conformational changes in the TMD and recruits downstream G proteins. In the absence of a ligand, weak ECD-TMD interactions inhibit receptor activation by preventing ligand binding, serving as a negative regulatory mechanism.
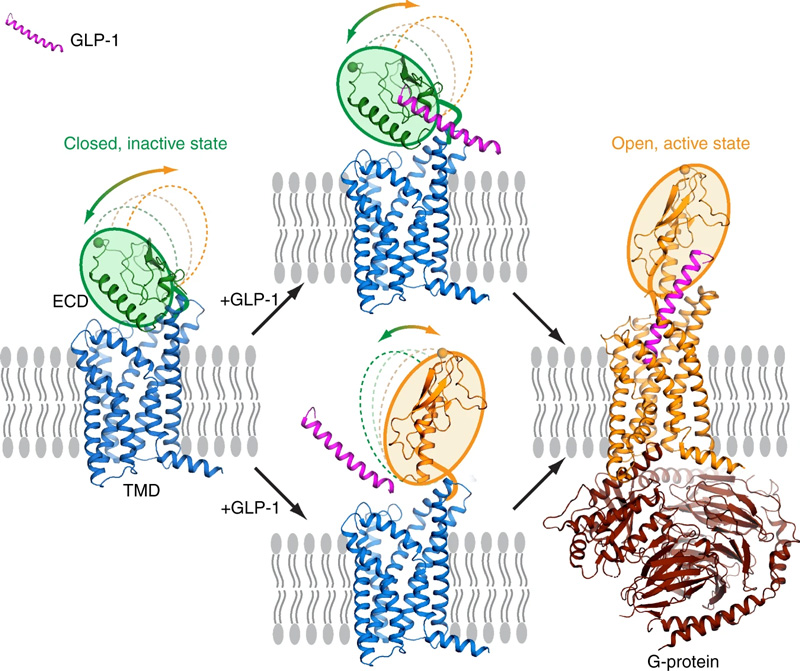
https://doi.org/10.1038/s41467-020-14934-5
Activation process of full-length GLP-1R
GLP-1R is widely distributed in the body, including in pancreatic beta cells, the stomach, intestines, brain, and heart. It regulates insulin secretion and blood glucose levels through its interaction with GLP-1. Additionally, GLP-1R influences appetite control, weight management, and provides cardiovascular and neuroprotective benefits, making it a key target for treating diabetes, obesity, and related diseases.
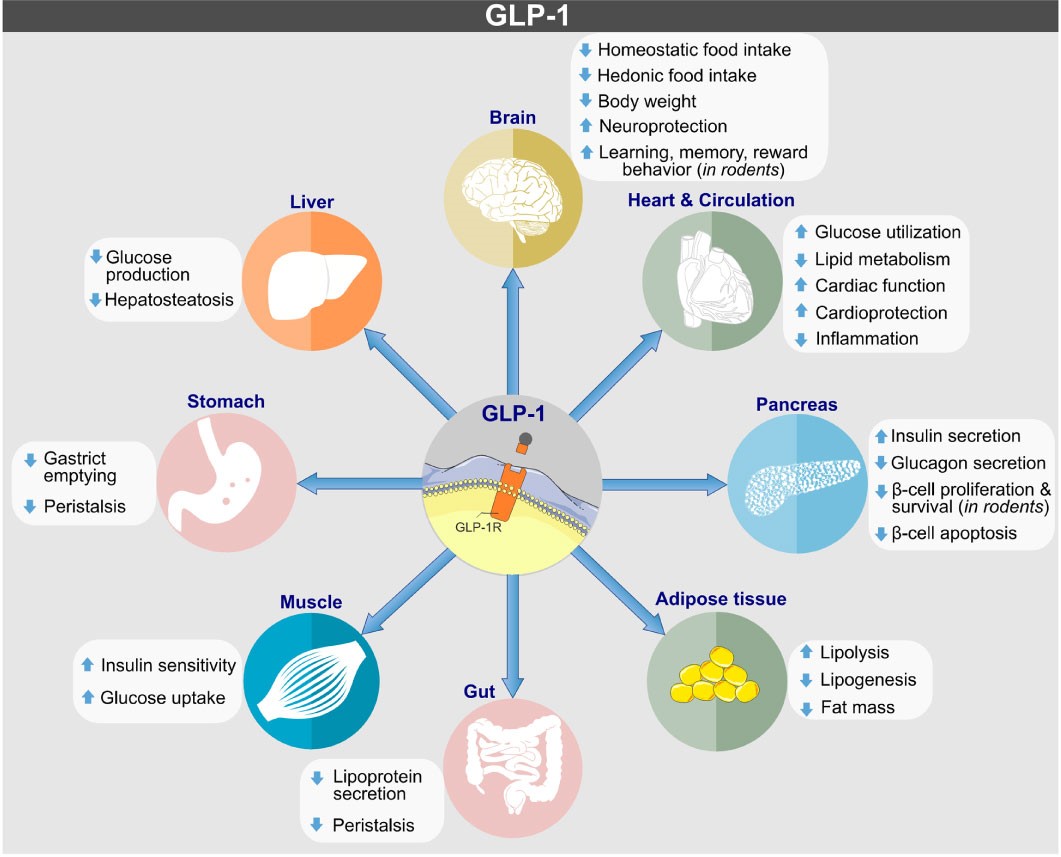
https://doi.org/10.3389/fendo.2022.838410
Biological actions of GLP-1 on target tissues
• Mainstream: Peptide-Based GLP-1R Drugs
Due to GLP-1R's dual role in regulating blood glucose and promoting weight loss, it has become a key target in diabetes treatments and aesthetic medicine. GLP-1R's high affinity for peptide ligands makes peptide-based drugs the primary form of GLP-1R-targeted therapies. However, the native GLP-1 molecule is rapidly degraded by dipeptidyl peptidase-4 (DPP-4) and glomerular filtration, requiring modifications to extend its half-life. To address this, several peptide-based GLP-1R agonists have been developed and approved, enhancing their half-life, efficacy, and bioavailability, effectively mimicking endogenous GLP-1's glucose-lowering and weight loss effects.
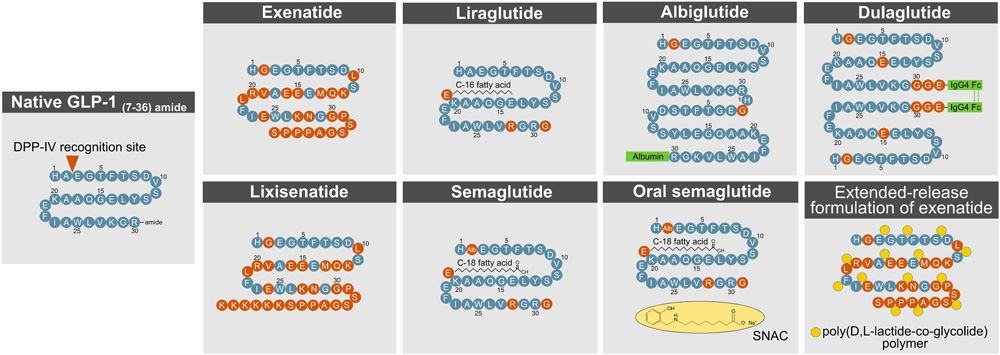
https://doi.org/10.3389/fendo.2022.838410
Methods to enhance GLP- 1R action
• Emerging: GLP-1R Antibody Drugs
As macromolecular drugs, antibody therapies offer higher specificity and affinity than peptide-based drugs, allowing precise targeting of antigens like GLP-1R and reducing the risk of nonspecific binding and side effects. Antibodies also benefit from extended circulation through the neonatal Fc receptor (FcRn), prolonging their half-life and reducing dosing frequency. Additionally, their immunomodulatory properties enhance efficacy via interactions with Fc receptors (FcR).
While peptide-based drugs often require weekly or more frequent administration, GLP-1R agonist antibodies offer a promising solution, with several under development to address this limitation.
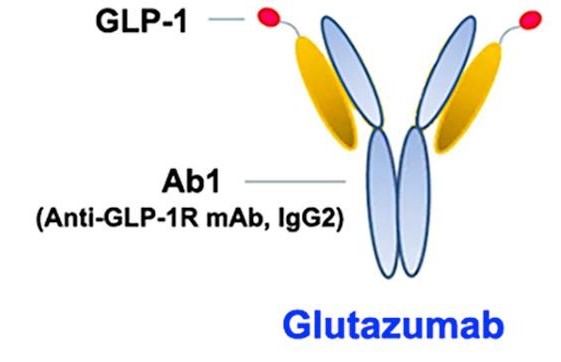
- Glutazumab by Gmax Biopharm: Glutazumab is the world's first antibody drug directly targeting GLP-1R, developed for treating type 2 diabetes and obesity. It features a fusion of a 23-amino-acid peptide linker and a DPP-4-resistant GLP-1 (7-35) fragment to the light chain of a humanized GLP-1R antibody. The most advanced clinical trial of Glutazumab has entered phase III. Existing clinical data indicate that Glutazumab is superior to similar GLP-1 analogs in terms of safety, tolerability, and glucose-lowering and weight loss effects, making it a potential next-generation ultra-long-acting treatment for diabetes and obesity.
https://doi.org/10.1016/j.bcp.2018.01.029
Schematic diagram of Glutazumab
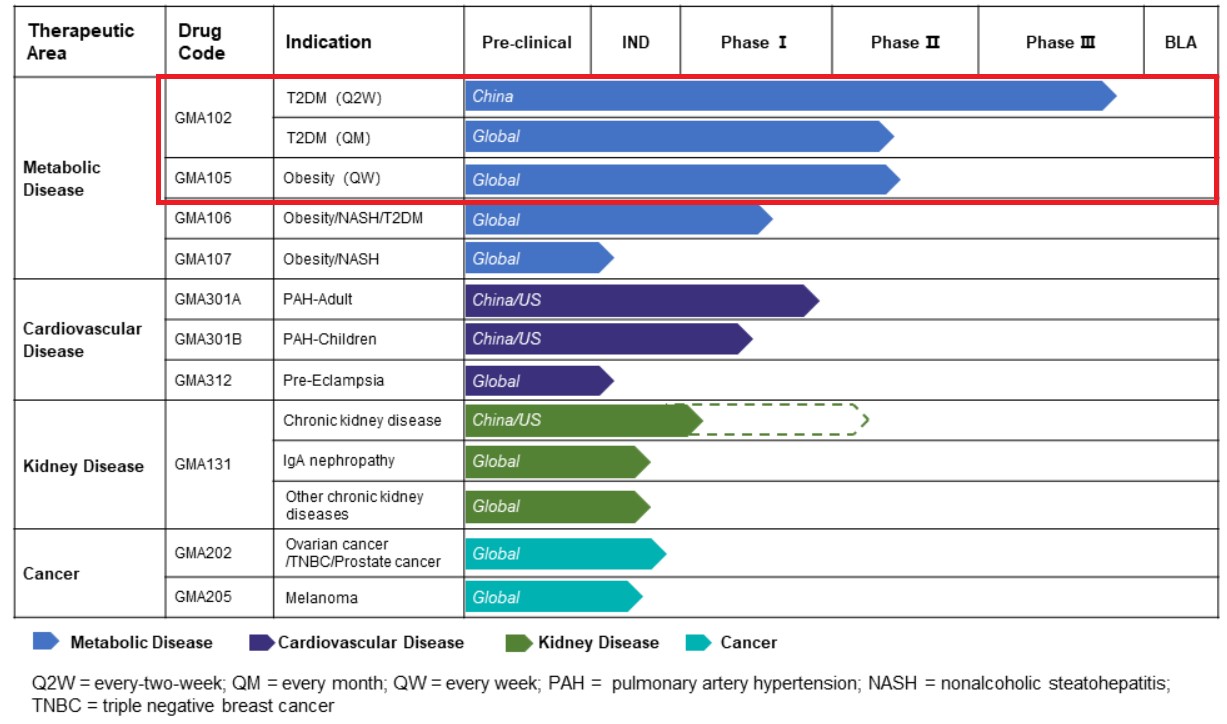
Source: Gmax Biopharm official website
The pipeline of Gmax Biopharm
- Regeneron's ATDC Technology: In September 2023, Regeneron patented (WO2023173132A1) antibody-tethered drug conjugates (ATDCs). This technology combines an antibody targeting the extracellular domain of GLP-1R with a GLP-1 mimetic, forming ATDCs. The GLP-1R antibody recognizes GLP-1R on the cell surface and inserts the modified GLP-1 peptide into the receptor, enhancing ligand binding. Compared to traditional peptide drugs, ATDCs increase affinity and molecular weight, improving the stability and half-life of GLP-1 peptide analogs.
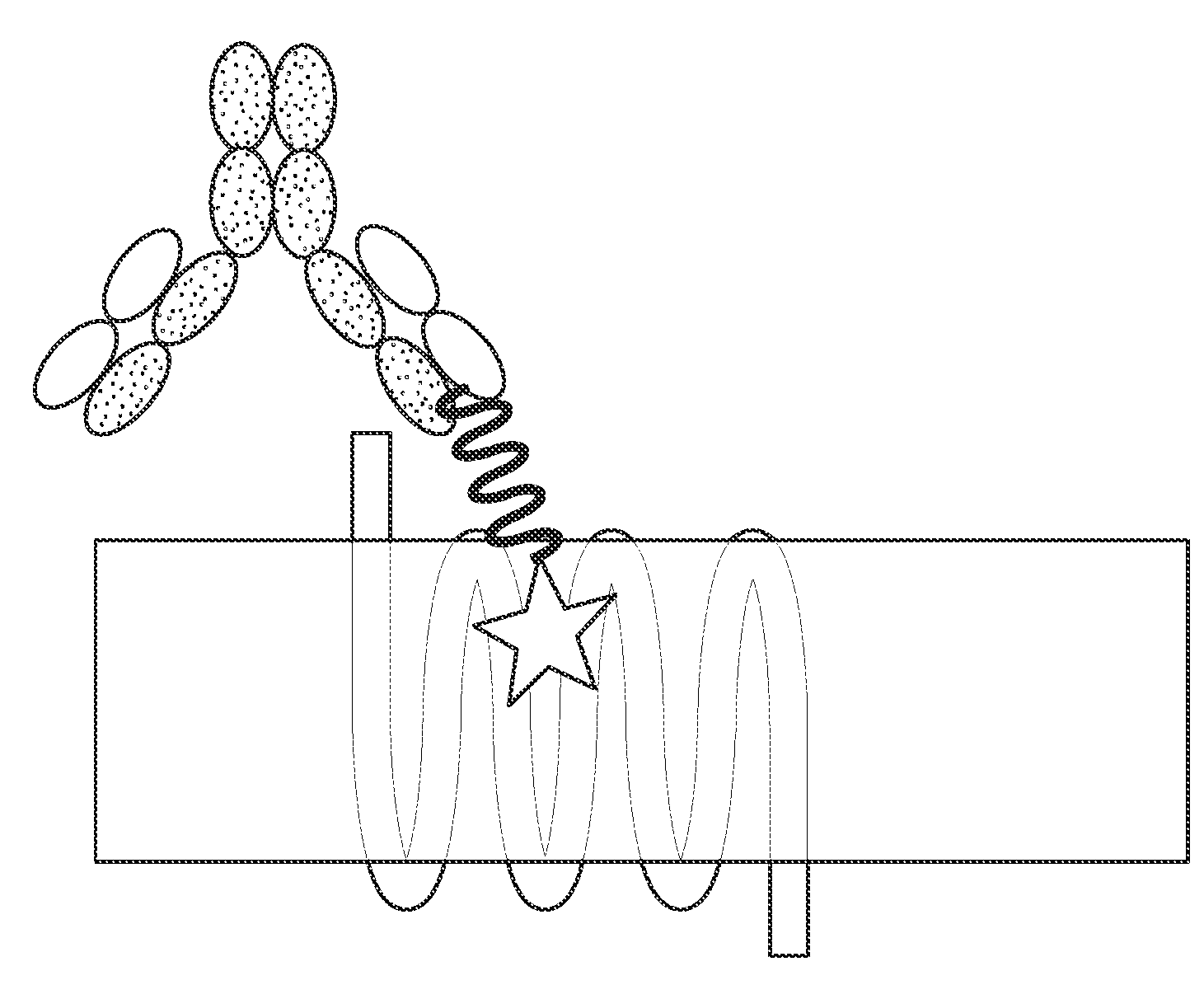
Source: Patent No. WO2023173132A1
The mechanism of ATDC
- Everestmab: Everestmab is an anti-GLP-1R antibody with agonistic effects achieved through fusion with a GLP-1 peptide segment. It has three domains: a mutated GLP-1 (A8G) fragment with enhanced DPP-4 resistance; a humanized human serum albumin (HSA) nanobody that binds HSA to extend half-life by preventing rapid clearance; and a humanized GLP-1R nanobody that activates the GLP-1R signaling pathway upon dissociation from HSA.
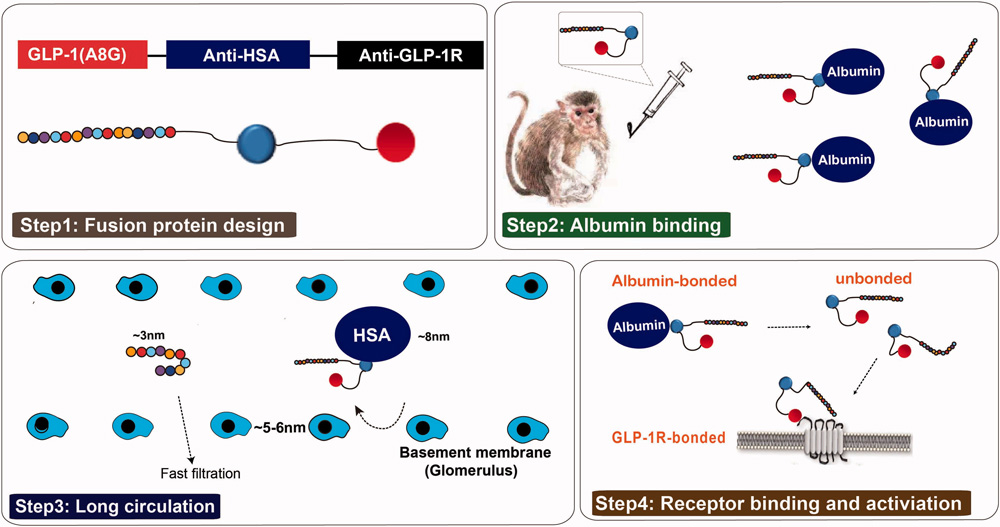
https://doi.org/10.1080/21691401.2020.1770268
Structure and the prolonged persistence mechanism of everestmab in vivo
- TB59-2 Antibody: Liu et al. used their GPCR synthetic antibody phage display library to identify the anti-GLP-1R antibody TB01-2, which has strong binding but lacks agonistic activity. By fusing the active GLP-1 (7-36) fragment to the N-terminus of TB01-2’s light chain, they created TB59-2. This fusion significantly enhances the antibody’s activity and stability, mimics endogenous GLP-1, and effectively activates GLP-1R, increasing insulin secretion and lowering blood glucose levels.
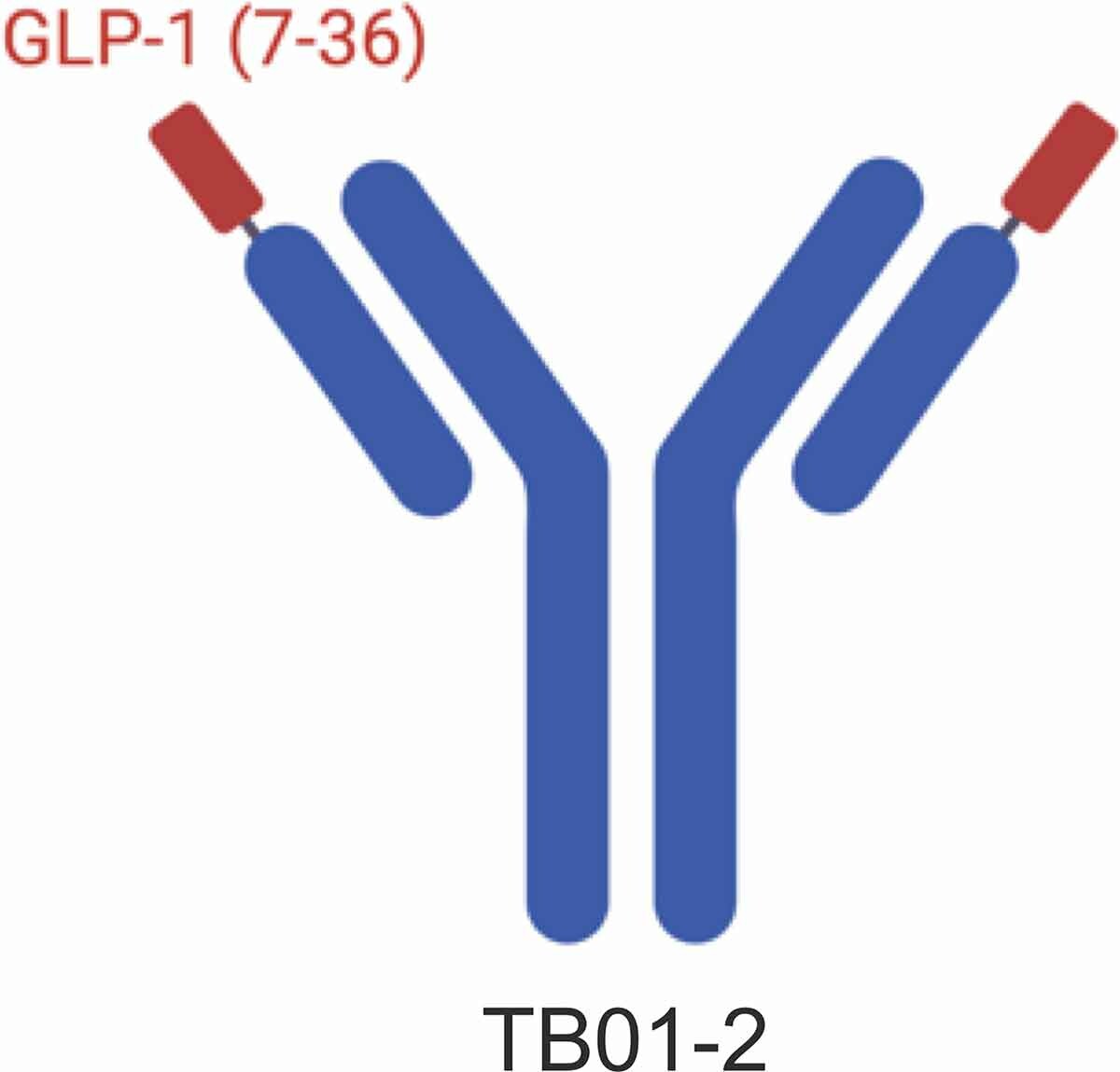
https://doi.org/10.1080/19420862.2021.1893425
Design of TB59-2
While peptide-based drugs lead in GLP-1R-targeted therapies, antibody-based GLP-1R drugs show significant clinical potential. To overcome challenges in expressing and obtaining the natural conformation of the seven-transmembrane GLP-1R, ACROBiosystems has developed a full-length GLP-1R protein via "FLAG". Expressed in the HEK293 system and validated through binding with GLP-1R agonists, this protein aids in developing GLP-1R-targeting antibody drugs.
1. Wu F, Yang L, Hang K, et al. Full-length human GLP-1 receptor structure without orthosteric ligands[J]. Nature communications, 2020, 11(1): 1272. https://doi.org/10.1038/s41467-020-14934-5
2. Tan Q, Akindehin S E, Orsso C E, et al. Recent advances in incretin-based pharmacotherapies for the treatment of obesity and diabetes[J]. Frontiers in Endocrinology, 2022, 13: 838410. https://doi.org/10.3389/fendo.2022.838410
3. Li C, Yang M, Wang X, et al. Glutazumab, a novel long-lasting GLP-1/anti-GLP-1R antibody fusion protein, exerts anti-diabetic effects through targeting dual receptor binding sites[J]. Biochemical Pharmacology, 2018, 150: 46-53. https://doi.org/10.1016/j.bcp.2018.01.029
4. Pan H, Su Y, **e Y, et al. Everestmab, a novel long-acting GLP-1/anti GLP-1R nanobody fusion protein, exerts potent anti-diabetic effects[J]. Artificial cells, nanomedicine, and Biotechnology, 2020, 48(1): 854-866. https://doi.org/10.1080/21691401.2020.1770268
5. Liu Q, Garg P, Hasdemir B, et al. Functional GLP-1R antibodies identified from a synthetic GPCR-focused library demonstrate potent blood glucose control[C]//MAbs. Taylor & Francis, 2021, 13(1): 1893425. https://doi.org/10.1080/19420862.2021.1893425
This web search service is supported by Google Inc.