 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| IFB-H5253 | Human | Human IFN-alpha 2b Protein, Fc Tag (MALS verified) |  |

|
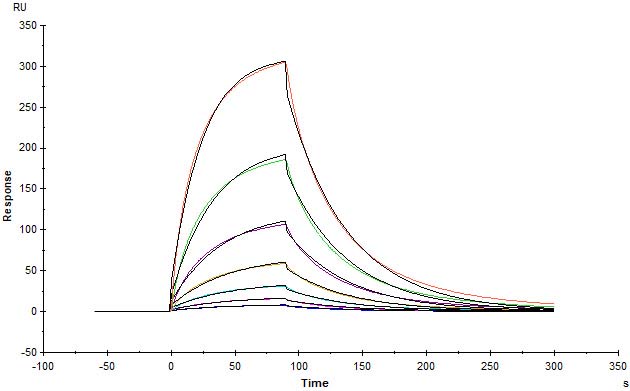
Human IFN-alpha 2b (K46R), Fc Tag (Cat. No. IFB-H5253) immobilized on CM5 Chip can bind Human IFNAR2, His Tag (Cat. No. IF2-H5224) with an affinity constant of 0.257 μM as determined in SPR assay (Biacore T200) (Routinely tested).
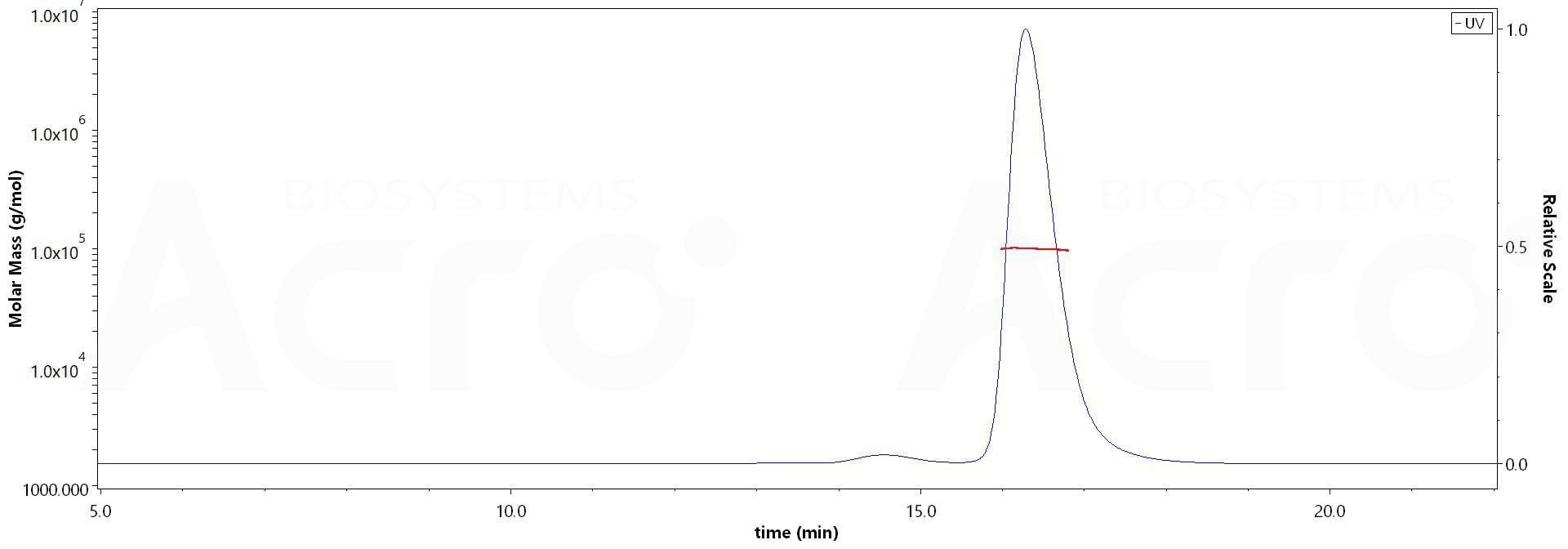
The purity of Human IFN-alpha 2b, Fc Tag (Cat. No. IFB-H5253) is more than 90% and the molecular weight of this protein is around 95-105 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Nadofaragene firadenovec | SCH-721015 | Approved | Schering - Plough Sa, Fkd Therapies | Adstiladrin | United States | Urinary Bladder Neoplasms | Ferring Pharmaceuticals A/S | 2022-12-16 | Mesothelioma; Urinary Bladder Neoplasms | Details |
| Nadofaragene firadenovec | SCH-721015 | Approved | Schering - Plough Sa, Fkd Therapies | Adstiladrin | United States | Urinary Bladder Neoplasms | Ferring Pharmaceuticals A/S | 2022-12-16 | Mesothelioma; Urinary Bladder Neoplasms | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Autologous CD34+-enriched hematopoietic stem and progenitor cells (Genenta Science) | Phase 2 Clinical | Genenta Science Srl | Glioblastoma; Multiple Myeloma | Details | |
| CX-801 | CX-801 | Phase 1 Clinical | Cytomx Therapeutics Inc | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Melanoma | Details |
| Autologous CD34+-enriched hematopoietic stem and progenitor cells (Genenta Science) | Phase 2 Clinical | Genenta Science Srl | Glioblastoma; Multiple Myeloma | Details | |
| CX-801 | CX-801 | Phase 1 Clinical | Cytomx Therapeutics Inc | Solid tumours; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Melanoma | Details |
This web search service is supported by Google Inc.