 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| Project Name | Project Stage | Molecule Type | Host Species | Therapeutic Area | Indications |
| GPRC5D VHH - 01 | PCC | Hematological Malignancy | Multiple myeloma |

FACS Analysis of CHO/Human GPRC5D Stable Cell Line.
FACS assay shows that Anti-GPRC5D antibody can bind to CHO/Human GPRC5D Stable Cell Line (Fig. B), and non-transfected CHO cells were used as a negative control (Fig. A). PE signal was used to evaluate the binding activity (QC tested).
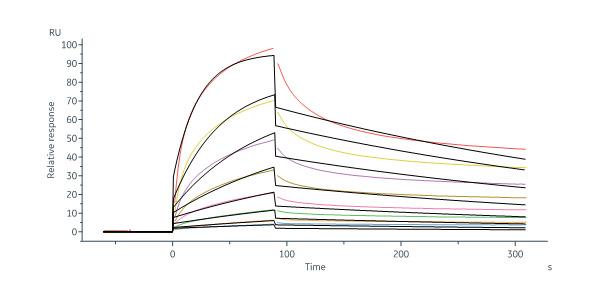
Anti-GPRC5D Antibody, Human IgG4 captured on Protein A Chip can bind Human GPRC5D Full Length Protein, Flag,His Tag (Cat. No. GPD-H52D5) with an affinity constant of 57.7 nM as determined in a SPR assay (in presence of DDM and CHS) (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Talquetamab | JNJ-7564; JNJ-64407564 | Approved | Johnson & Johnson Innovative Medicine, Genmab A/S | TALVEY | United States | Multiple Myeloma | Janssen Biotech Inc | 2023-08-09 | Hematologic Neoplasms; Multiple Myeloma | Details |
| Talquetamab | JNJ-7564; JNJ-64407564 | Approved | Johnson & Johnson Innovative Medicine, Genmab A/S | TALVEY | United States | Multiple Myeloma | Janssen Biotech Inc | 2023-08-09 | Hematologic Neoplasms; Multiple Myeloma | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| LM-305 | LM-305 | Phase 2 Clinical | LaNova Medicines Ltd | Multiple Myeloma | Details |
| Anti-GPRC5D CAR-T Cells Therapy (Shanghai YaKe Biotechnology) | Phase 2 Clinical | Shanghai YaKe Biotechnology Co Ltd | Multiple Myeloma | Details | |
| IBI-3003 | IBI3003; IBI-3003 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Multiple Myeloma | Details |
| BCMA-GPRC5D CAR-T Cells Therapy(Wuhan Union Hospital) | Phase 2 Clinical | Guangzhou Bio-Gene Technology Co Ltd, Wuhan Union Hospital | Multiple Myeloma | Details | |
| MBS-314 | MBS-314 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Multiple Myeloma | Details |
| CT-071 | CT071; CT-071 | Phase 2 Clinical | CARsgen Therapeutics Holdings Ltd | Leukemia, Plasma Cell; Multiple Myeloma | Details |
| Anti-GPRC5D CAR-T Cells Therapy(920th Hospital) | Phase 2 Clinical | The 920th Hospital Of Joint Logistics Support Force Of PLA | Multiple Myeloma | Details | |
| Ori-CAR-017 | Ori-CAR-017; OriCAR-017; GPRC5D-CAR-T | Phase 2 Clinical | OriCell Therapeutics Co Ltd | Hematologic Diseases; Hemorrhagic Disorders; Immunoproliferative Disorders; Hemostatic Disorders; Paraproteinemias; Neoplasms; Blood Protein Disorders; Multiple Myeloma; Immune System Diseases; Vascular Diseases; Neoplasms, Plasma Cell; Cardiovascular Diseases; Lymphoproliferative Disorders | Details |
| LBL-034 | LBL-034 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Neoplasms; Multiple Myeloma | Details |
| CC-95266 | CC-95266; BMS986393; BMS-986393 | Phase 2 Clinical | Juno Therapeutics Inc | Multiple Myeloma | Details |
| BCMAxGPRC5D CAR-T therapy (Juno Therapeutics) | BMS-986453 | Phase 1 Clinical | Juno Therapeutics Inc | Multiple Myeloma | Details |
| SIM-0500 | SIM-0500; SIM0500 | Phase 1 Clinical | Hainan Xiansheng Re Ming Pharmaceutical Co Ltd | Bone Marrow Neoplasms; Multiple Myeloma | Details |
| OriC-321 | OriC-321; Ori-C-321 | Phase 1 Clinical | Zhejiang University, OriCell Therapeutics Co Ltd | Multiple Myeloma | Details |
| CAR-GPRC5D | CAR-GPRC5D | Phase 1 Clinical | Nanjing Iaso Biotherapeutics Co Ltd | Leukemia, Plasma Cell; Multiple Myeloma | Details |
| Forimtamig | RG-6234 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| MCARH-109 | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center, Juno Therapeutics Inc, Eureka Therapeutics Inc | Multiple Myeloma | Details | |
| RO-7425781 | RO-7425781 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| Anti-GPRC5D-CD19-CAR-T cell therapy(The Second People Hospital Of Guangdong Province) | The Second People Hospital Of Guangdong Province | Details | |||
| Anti-GPRC5D CAR-T Cells Therapy(Institute of Hematology & Blood Diseases Hospital) | Institute Of Hematology & Blood Diseases Hospital | Details | |||
| LM-305 | LM-305 | Phase 2 Clinical | LaNova Medicines Ltd | Multiple Myeloma | Details |
| Anti-GPRC5D CAR-T Cells Therapy (Shanghai YaKe Biotechnology) | Phase 2 Clinical | Shanghai YaKe Biotechnology Co Ltd | Multiple Myeloma | Details | |
| IBI-3003 | IBI3003; IBI-3003 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Multiple Myeloma | Details |
| BCMA-GPRC5D CAR-T Cells Therapy(Wuhan Union Hospital) | Phase 2 Clinical | Guangzhou Bio-Gene Technology Co Ltd, Wuhan Union Hospital | Multiple Myeloma | Details | |
| MBS-314 | MBS-314 | Phase 2 Clinical | Beijing Mabworks Biotech Co Ltd | Multiple Myeloma | Details |
| CT-071 | CT071; CT-071 | Phase 2 Clinical | CARsgen Therapeutics Holdings Ltd | Leukemia, Plasma Cell; Multiple Myeloma | Details |
| Anti-GPRC5D CAR-T Cells Therapy(920th Hospital) | Phase 2 Clinical | The 920th Hospital Of Joint Logistics Support Force Of PLA | Multiple Myeloma | Details | |
| Ori-CAR-017 | Ori-CAR-017; OriCAR-017; GPRC5D-CAR-T | Phase 2 Clinical | OriCell Therapeutics Co Ltd | Hematologic Diseases; Hemorrhagic Disorders; Immunoproliferative Disorders; Hemostatic Disorders; Paraproteinemias; Neoplasms; Blood Protein Disorders; Multiple Myeloma; Immune System Diseases; Vascular Diseases; Neoplasms, Plasma Cell; Cardiovascular Diseases; Lymphoproliferative Disorders | Details |
| LBL-034 | LBL-034 | Phase 2 Clinical | Nanjing Leads Biolabs Co Ltd | Neoplasms; Multiple Myeloma | Details |
| CC-95266 | CC-95266; BMS986393; BMS-986393 | Phase 2 Clinical | Juno Therapeutics Inc | Multiple Myeloma | Details |
| BCMAxGPRC5D CAR-T therapy (Juno Therapeutics) | BMS-986453 | Phase 1 Clinical | Juno Therapeutics Inc | Multiple Myeloma | Details |
| SIM-0500 | SIM-0500; SIM0500 | Phase 1 Clinical | Hainan Xiansheng Re Ming Pharmaceutical Co Ltd | Bone Marrow Neoplasms; Multiple Myeloma | Details |
| OriC-321 | OriC-321; Ori-C-321 | Phase 1 Clinical | Zhejiang University, OriCell Therapeutics Co Ltd | Multiple Myeloma | Details |
| CAR-GPRC5D | CAR-GPRC5D | Phase 1 Clinical | Nanjing Iaso Biotherapeutics Co Ltd | Leukemia, Plasma Cell; Multiple Myeloma | Details |
| Forimtamig | RG-6234 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| MCARH-109 | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center, Juno Therapeutics Inc, Eureka Therapeutics Inc | Multiple Myeloma | Details | |
| RO-7425781 | RO-7425781 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Multiple Myeloma | Details |
| Anti-GPRC5D-CD19-CAR-T cell therapy(The Second People Hospital Of Guangdong Province) | The Second People Hospital Of Guangdong Province | Details | |||
| Anti-GPRC5D CAR-T Cells Therapy(Institute of Hematology & Blood Diseases Hospital) | Institute Of Hematology & Blood Diseases Hospital | Details |
This web search service is supported by Google Inc.