 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
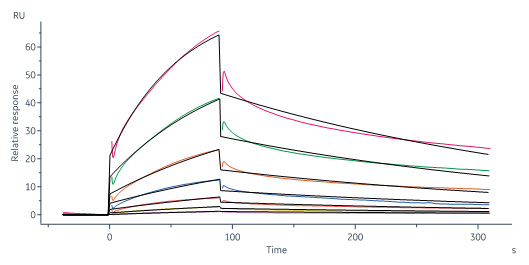
Human MAdCAM-1, His Tag (Cat. No. MAM-H52H4) immobilized on CM5 Chip can bind Human ITGA4&ITGB7 Heterodimer Protein, His Tag&Tag Free (Cat. No. IT7-H52W4) with an affinity constant of 0.227 μM as determined in a SPR assay (Biacore 8K) (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Vedolizumab | MLN-0002; LPD-02; MLN-002; MLN-02; LDP-02 | Approved | Millennium Pharmaceuticals Inc | 安吉优, Entyvio | United States | Colitis, Ulcerative; Crohn Disease | Takeda Pharmaceuticals U.S.A. Inc | 2014-05-20 | Colitis; Melanoma; Carcinoma, Non-Small-Cell Lung; Crohn Disease; Urogenital Neoplasms; Colitis, Ulcerative; Pediatric ulcerative colitis; Celiac Disease; Cholangitis, Sclerosing; Solid tumours; Neoplasms; Pouchitis; Pediatric Crohn's disease; Inflammatory Bowel Diseases; Acquired Immunodeficiency Syndrome; Hematopoietic stem cell transplantation (HSCT); HIV Infections | Details |
| Vedolizumab | MLN-0002; LPD-02; MLN-002; MLN-02; LDP-02 | Approved | Millennium Pharmaceuticals Inc | 安吉优, Entyvio | United States | Colitis, Ulcerative; Crohn Disease | Takeda Pharmaceuticals U.S.A. Inc | 2014-05-20 | Colitis; Melanoma; Carcinoma, Non-Small-Cell Lung; Crohn Disease; Urogenital Neoplasms; Colitis, Ulcerative; Pediatric ulcerative colitis; Celiac Disease; Cholangitis, Sclerosing; Solid tumours; Neoplasms; Pouchitis; Pediatric Crohn's disease; Inflammatory Bowel Diseases; Acquired Immunodeficiency Syndrome; Hematopoietic stem cell transplantation (HSCT); HIV Infections | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Ontamalimab | SHP-647; PF-00547659; PF-547659 | Phase 3 Clinical | Pfizer Inc | Ileitis; Colitis, Ulcerative; Crohn Disease | Details |
| Vedolizumab biosimilar (Polpharma Biologics) | PB-016 | Phase 3 Clinical | Polpharma Biologics Sa | Pouchitis; Colitis, Ulcerative; Crohn Disease | Details |
| Ontamalimab | SHP-647; PF-00547659; PF-547659 | Phase 3 Clinical | Pfizer Inc | Ileitis; Colitis, Ulcerative; Crohn Disease | Details |
| Vedolizumab biosimilar (Polpharma Biologics) | PB-016 | Phase 3 Clinical | Polpharma Biologics Sa | Pouchitis; Colitis, Ulcerative; Crohn Disease | Details |
This web search service is supported by Google Inc.