 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| DDC-H55H6 | Human | Human DDC / Dopa Decarboxilase Protein, His Tag (MALS verified) |  |

|
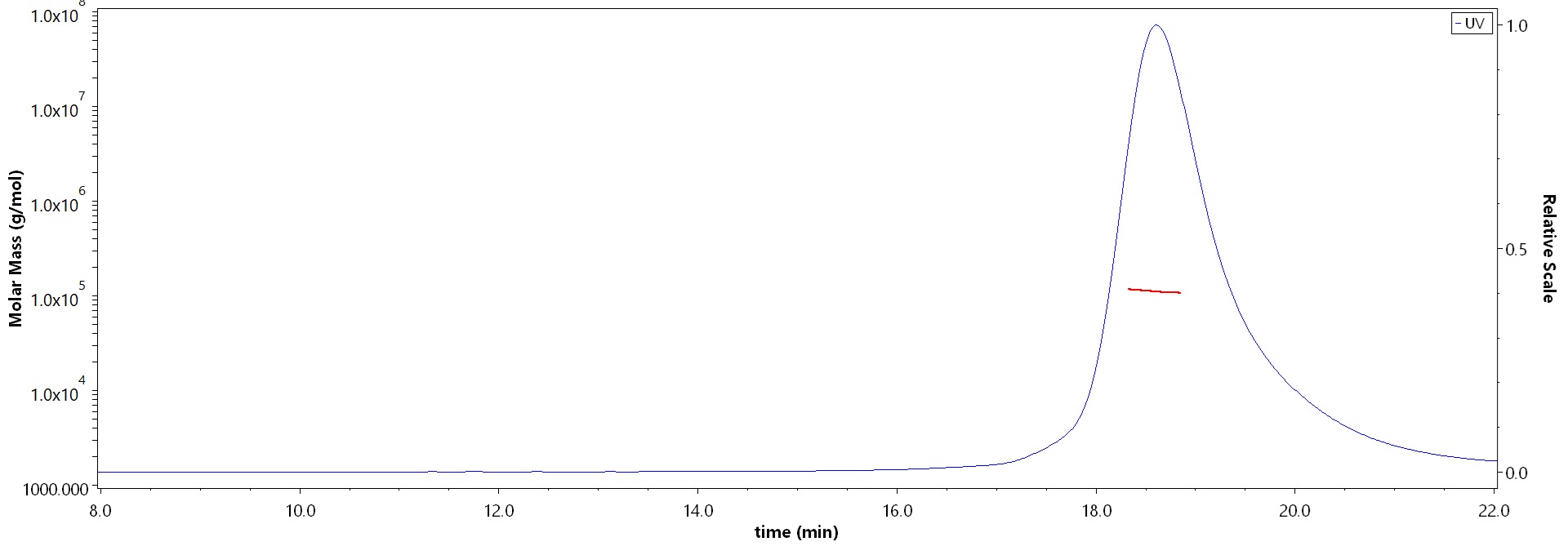
The purity of Human DDC, His Tag (Cat. No. DDC-H55H6) is more than 90% and the molecular weight of this protein is around 100-125 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Levodopa/Benserazide Hydrochloride | Approved | F. Hoffmann-La Roche Ltd | Madopar | Switzerland | Parkinson Disease | F. Hoffmann-La Roche Ltd | 1973-06-27 | Parkinson Disease | Details | |
| Eladocagene exuparvovec | PTC-AADC | Approved | National Taiwan University, Ptc Therapeutics Inc | Upstaza | EU | Amino Acid Metabolism, Inborn Errors | Ptc Therapeutics International Ltd | 2022-07-18 | Aromatic amino acid decarboxylase deficiency; Amino Acid Metabolism, Inborn Errors | Details |
| Levodopa/Carbidopa | IPX-066; ABT-SLV-187; ND-0680; ND-0612L; AP-CD/LD; ND-0612; IPX-054; ABT-SLV187; IPX-203; CP-012; AP-09004; DM-1992; AP-CDLD; TPI-926; ND-0612H; PW-4153; LCIG; JM-012; SOL-707; INP-107(POD™ carbidopa/levodopa) | Approved | Merck Sharp & Dohme Corp | Numient, Menesit, Duodopa, Vadova, Sinemet, Rytary, Isicom, Duopa, Flexilev, Nacom (imciromab), Nacom retard, Parcopa, DopaFuse, Patrome | United States | Parkinson Disease; Parkinson Disease, Secondary; Parkinson Disease, Postencephalitic | Merck & Co Inc | 1975-05-02 | Albinism, Oculocutaneous; Diabetes Mellitus; Amyotrophic Lateral Sclerosis; Diabetic Retinopathy; Albinism; Cocaine-Related Disorders; Amblyopia; Depressive Disorder, Treatment-Resistant; Cognitive Dysfunction; Motor Neuron Disease; Aphasia, Broca; Parkinson Disease; Stroke; Parkinson Disease, Postencephalitic; Depression; Parkinson Disease, Secondary; Gait Apraxia | Details |
| Benserazide Hydrochloride | Ro-4-4602 | Approved | Mainland China | Parkinson Disease; Dyskinesias | Shanghai Yishengyuan Pharmaceutical Co Ltd | 1995-01-01 | beta-Thalassemia; Dyskinesias; Parkinson Disease; Anemia, Sickle Cell | Details | ||
| Foslevodopa/Foscarbidopa | ABBV-951 | Approved | Abbvie Inc | VYALEV, Vyalev, Duodopa, Produodopa | Romania, Belgium, Cyprus | Parkinson Disease | Abbvie Deutschland Gmbh & Co Kg, Abbvie Sa, Abbvie Inc | Parkinson Disease | Details | |
| Carbidopa/Entacapone/Levodopa | ELC-200; ODM-101 | Approved | Orion Pharma | Stalevo, LECIGon, Trigel, LECIGel, 达灵复 | United States | Parkinson Disease | Orion Pharma A/S | 2003-06-11 | Parkinson Disease | Details |
| Carbidopa | MK-486 | Approved | Lodosyn | United States | Parkinson Disease | Aton | 1977-04-25 | Postural Orthostatic Tachycardia Syndrome; Parkinson Disease; Orthostatic Intolerance | Details | |
| Carbidopa/Melevodopa | V-1512; GT-1512; CHF-1512; CNP-1512 | Approved | Chiesi Farmaceutici SPA | Sirio | Italy | Parkinson Disease | null | 2003-01-01 | Parkinson Disease | Details |
| Levodopa/Benserazide Hydrochloride | Approved | F. Hoffmann-La Roche Ltd | Madopar | Switzerland | Parkinson Disease | F. Hoffmann-La Roche Ltd | 1973-06-27 | Parkinson Disease | Details | |
| Eladocagene exuparvovec | PTC-AADC | Approved | National Taiwan University, Ptc Therapeutics Inc | Upstaza | EU | Amino Acid Metabolism, Inborn Errors | Ptc Therapeutics International Ltd | 2022-07-18 | Aromatic amino acid decarboxylase deficiency; Amino Acid Metabolism, Inborn Errors | Details |
| Levodopa/Carbidopa | IPX-066; ABT-SLV-187; ND-0680; ND-0612L; AP-CD/LD; ND-0612; IPX-054; ABT-SLV187; IPX-203; CP-012; AP-09004; DM-1992; AP-CDLD; TPI-926; ND-0612H; PW-4153; LCIG; JM-012; SOL-707; INP-107(POD™ carbidopa/levodopa) | Approved | Merck Sharp & Dohme Corp | Numient, Menesit, Duodopa, Vadova, Sinemet, Rytary, Isicom, Duopa, Flexilev, Nacom (imciromab), Nacom retard, Parcopa, DopaFuse, Patrome | United States | Parkinson Disease; Parkinson Disease, Secondary; Parkinson Disease, Postencephalitic | Merck & Co Inc | 1975-05-02 | Albinism, Oculocutaneous; Diabetes Mellitus; Amyotrophic Lateral Sclerosis; Diabetic Retinopathy; Albinism; Cocaine-Related Disorders; Amblyopia; Depressive Disorder, Treatment-Resistant; Cognitive Dysfunction; Motor Neuron Disease; Aphasia, Broca; Parkinson Disease; Stroke; Parkinson Disease, Postencephalitic; Depression; Parkinson Disease, Secondary; Gait Apraxia | Details |
| Benserazide Hydrochloride | Ro-4-4602 | Approved | Mainland China | Parkinson Disease; Dyskinesias | Shanghai Yishengyuan Pharmaceutical Co Ltd | 1995-01-01 | beta-Thalassemia; Dyskinesias; Parkinson Disease; Anemia, Sickle Cell | Details | ||
| Foslevodopa/Foscarbidopa | ABBV-951 | Approved | Abbvie Inc | VYALEV, Vyalev, Duodopa, Produodopa | Romania, Belgium, Cyprus | Parkinson Disease | Abbvie Deutschland Gmbh & Co Kg, Abbvie Sa, Abbvie Inc | Parkinson Disease | Details | |
| Carbidopa/Entacapone/Levodopa | ELC-200; ODM-101 | Approved | Orion Pharma | Stalevo, LECIGon, Trigel, LECIGel, 达灵复 | United States | Parkinson Disease | Orion Pharma A/S | 2003-06-11 | Parkinson Disease | Details |
| Carbidopa | MK-486 | Approved | Lodosyn | United States | Parkinson Disease | Aton | 1977-04-25 | Postural Orthostatic Tachycardia Syndrome; Parkinson Disease; Orthostatic Intolerance | Details | |
| Carbidopa/Melevodopa | V-1512; GT-1512; CHF-1512; CNP-1512 | Approved | Chiesi Farmaceutici SPA | Sirio | Italy | Parkinson Disease | null | 2003-01-01 | Parkinson Disease | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Buspirone/Carbidopa/Levodopa | Phase 2 Clinical | Universite Laval | Spinal Cord Injuries; Multiple Sclerosis | Details | |
| Levodopa/Carbidopa controlled release tablets-night(WD Pharmaceutical) | WD-1905 | Phase 2 Clinical | Shanghai WD Pharmaceutical Co Ltd | Parkinsonian Disorders | Details |
| Levodopa/Carbidopa controlled release tablets(WD Pharmaceutical) | WD-1603 | Phase 2 Clinical | Shanghai WD Pharmaceutical Co Ltd | Parkinsonian Disorders | Details |
| Levodopa/Carbidopa (Hengrui Medicine) | HRG-2010 | Phase 2 Clinical | Jiangsu Hengrui Medicine Co Ltd | Parkinson Disease | Details |
| Ezaladcigene resoparvovec | VY-AADC; VY-AADC01; GZ-404477; AV-201; VY-AADC02 | Phase 1 Clinical | University Of California, Voyager Therapeutics Inc | Aromatic amino acid decarboxylase deficiency; Parkinson Disease | Details |
| Carbidopa/oxytriptan | EVX-101 | Phase 1 Clinical | Evecxia Therapeutics Inc | Depression | Details |
| VGN-R09 | VGN-R09b; VGN-R09 | Phase 1 Clinical | Shanghai Vitalgen BioPharma Co Ltd | Aromatic amino acid decarboxylase deficiency | Details |
| Levodopa/Carbidopa granule(WD Pharmaceutical) | WD-2010 | Phase 1 Clinical | Shanghai WD Pharmaceutical Co Ltd | Parkinsonian Disorders | Details |
| Levodopa/Carbidopa(WD Pharmaceutical) | WD-1804 | Phase 1 Clinical | Shanghai WD Pharmaceutical Co Ltd | Parkinsonian Disorders | Details |
| Buspirone/Carbidopa/Levodopa | Phase 2 Clinical | Universite Laval | Spinal Cord Injuries; Multiple Sclerosis | Details | |
| Levodopa/Carbidopa controlled release tablets-night(WD Pharmaceutical) | WD-1905 | Phase 2 Clinical | Shanghai WD Pharmaceutical Co Ltd | Parkinsonian Disorders | Details |
| Levodopa/Carbidopa controlled release tablets(WD Pharmaceutical) | WD-1603 | Phase 2 Clinical | Shanghai WD Pharmaceutical Co Ltd | Parkinsonian Disorders | Details |
| Levodopa/Carbidopa (Hengrui Medicine) | HRG-2010 | Phase 2 Clinical | Jiangsu Hengrui Medicine Co Ltd | Parkinson Disease | Details |
| Ezaladcigene resoparvovec | VY-AADC; VY-AADC01; GZ-404477; AV-201; VY-AADC02 | Phase 1 Clinical | University Of California, Voyager Therapeutics Inc | Aromatic amino acid decarboxylase deficiency; Parkinson Disease | Details |
| Carbidopa/oxytriptan | EVX-101 | Phase 1 Clinical | Evecxia Therapeutics Inc | Depression | Details |
| VGN-R09 | VGN-R09b; VGN-R09 | Phase 1 Clinical | Shanghai Vitalgen BioPharma Co Ltd | Aromatic amino acid decarboxylase deficiency | Details |
| Levodopa/Carbidopa granule(WD Pharmaceutical) | WD-2010 | Phase 1 Clinical | Shanghai WD Pharmaceutical Co Ltd | Parkinsonian Disorders | Details |
| Levodopa/Carbidopa(WD Pharmaceutical) | WD-1804 | Phase 1 Clinical | Shanghai WD Pharmaceutical Co Ltd | Parkinsonian Disorders | Details |
This web search service is supported by Google Inc.