 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| Project Name | Modality | Therapeutic Area | Indications | Stage | Right Available |
| Reversible MAOB Inhibitor | Small molecule | Neurological disease | Parkinson's disease | Phase I | Global |
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| MAB-H5547 | Human | Human MAOB / Monoamine Oxidase B Protein, His Tag (active enzyme) |  |
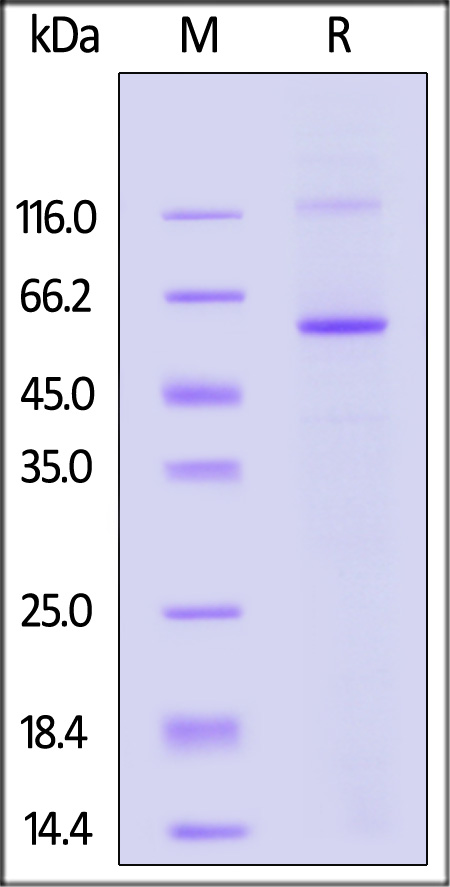
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Rasagiline Mesylate | Lu 00-773; TVP-101; TV-1030; AGN-1135; TVP-1012 | Approved | Teva | Azilect, Agilect | EU | Parkinson Disease | Teva Bv | 2005-02-21 | Supranuclear Palsy, Progressive; Restless Legs Syndrome; Parkinson Disease; Dementia; Alzheimer Disease; Synucleinopathies; Multiple System Atrophy; REM Sleep Behavior Disorder | Details |
| Selegiline | Approved | Mylan Nv, Somerset Inc | Emsam | United States | Depressive Disorder, Major | Somerset Pharmaceuticals Inc | 2006-02-27 | Tobacco Use Cessation; Depressive Disorder, Major; Tobacco Use Disorder; Amphetamine-Related Disorders; Cocaine-Related Disorders | Details | |
| Selegiline Hydrochloride | FPF-1100; E-250 (free base) | Approved | Sanofi | FP, Jumex, Otrasel, Seledat, Xilopar, Zelapar, Deprenyl, Eldepryl, Plurimen, Vivapryl, Zydis selegiline | Mainland China | Parkinson Disease | Chinoin Pharmaceutical And Chemical Works Co Ltd | 1989-06-05 | HIV Infections; Marijuana Abuse; Parkinson Disease; Cognition Disorders | Details |
| Safinamide Methanesulfonate | PNU-151774; FCE-26743; PNU-151774E; FCE-28073 (R-isomer); EMD-1195686; ZP-034; NW-1015; ME-2125 | Approved | Newron Pharmaceutical | Xadago, Equfina | EU | Parkinson Disease | Zambon Spa | 2015-02-23 | Kidney Diseases; Parkinson Disease; Hepatic Insufficiency; Dyskinesia, Drug-Induced; Multiple System Atrophy | Details |
| Rasagiline Mesylate | Lu 00-773; TVP-101; TV-1030; AGN-1135; TVP-1012 | Approved | Teva | Azilect, Agilect | EU | Parkinson Disease | Teva Bv | 2005-02-21 | Supranuclear Palsy, Progressive; Restless Legs Syndrome; Parkinson Disease; Dementia; Alzheimer Disease; Synucleinopathies; Multiple System Atrophy; REM Sleep Behavior Disorder | Details |
| Selegiline | Approved | Mylan Nv, Somerset Inc | Emsam | United States | Depressive Disorder, Major | Somerset Pharmaceuticals Inc | 2006-02-27 | Tobacco Use Cessation; Depressive Disorder, Major; Tobacco Use Disorder; Amphetamine-Related Disorders; Cocaine-Related Disorders | Details | |
| Selegiline Hydrochloride | FPF-1100; E-250 (free base) | Approved | Sanofi | FP, Jumex, Otrasel, Seledat, Xilopar, Zelapar, Deprenyl, Eldepryl, Plurimen, Vivapryl, Zydis selegiline | Mainland China | Parkinson Disease | Chinoin Pharmaceutical And Chemical Works Co Ltd | 1989-06-05 | HIV Infections; Marijuana Abuse; Parkinson Disease; Cognition Disorders | Details |
| Safinamide Methanesulfonate | PNU-151774; FCE-26743; PNU-151774E; FCE-28073 (R-isomer); EMD-1195686; ZP-034; NW-1015; ME-2125 | Approved | Newron Pharmaceutical | Xadago, Equfina | EU | Parkinson Disease | Zambon Spa | 2015-02-23 | Kidney Diseases; Parkinson Disease; Hepatic Insufficiency; Dyskinesia, Drug-Induced; Multiple System Atrophy | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Pramipexole Dihydrochloride Hydrate/Rasagiline Mesylate | P2B-001 | Phase 3 Clinical | Pharma Two B Ltd | Parkinson Disease | Details |
| Vafidemstat | ORY-2001 | Phase 2 Clinical | Oryzon Genomics Sa | Schizophrenia; Alzheimer Disease; Kabuki syndrome; Borderline Personality Disorder | Details |
| Rasagiline transdermal patch (Teikoku Pharma) | Phase 1 Clinical | Teikoku Pharma | Parkinson Disease | Details | |
| J-147 | J-147 | Phase 1 Clinical | Salk Institute | Alzheimer Disease | Details |
| SKL-PD | YKP-10461; SKL-PD | Phase 1 Clinical | SK Biopharmaceuticals Co Ltd | Parkinson Disease | Details |
| PXS-5131 | PXS-5131 | Innovent Biologics(Suzhou) Co Ltd | Details | ||
| Pramipexole Dihydrochloride Hydrate/Rasagiline Mesylate | P2B-001 | Phase 3 Clinical | Pharma Two B Ltd | Parkinson Disease | Details |
| Vafidemstat | ORY-2001 | Phase 2 Clinical | Oryzon Genomics Sa | Schizophrenia; Alzheimer Disease; Kabuki syndrome; Borderline Personality Disorder | Details |
| Rasagiline transdermal patch (Teikoku Pharma) | Phase 1 Clinical | Teikoku Pharma | Parkinson Disease | Details | |
| J-147 | J-147 | Phase 1 Clinical | Salk Institute | Alzheimer Disease | Details |
| SKL-PD | YKP-10461; SKL-PD | Phase 1 Clinical | SK Biopharmaceuticals Co Ltd | Parkinson Disease | Details |
| PXS-5131 | PXS-5131 | Innovent Biologics(Suzhou) Co Ltd | Details |
This web search service is supported by Google Inc.