 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
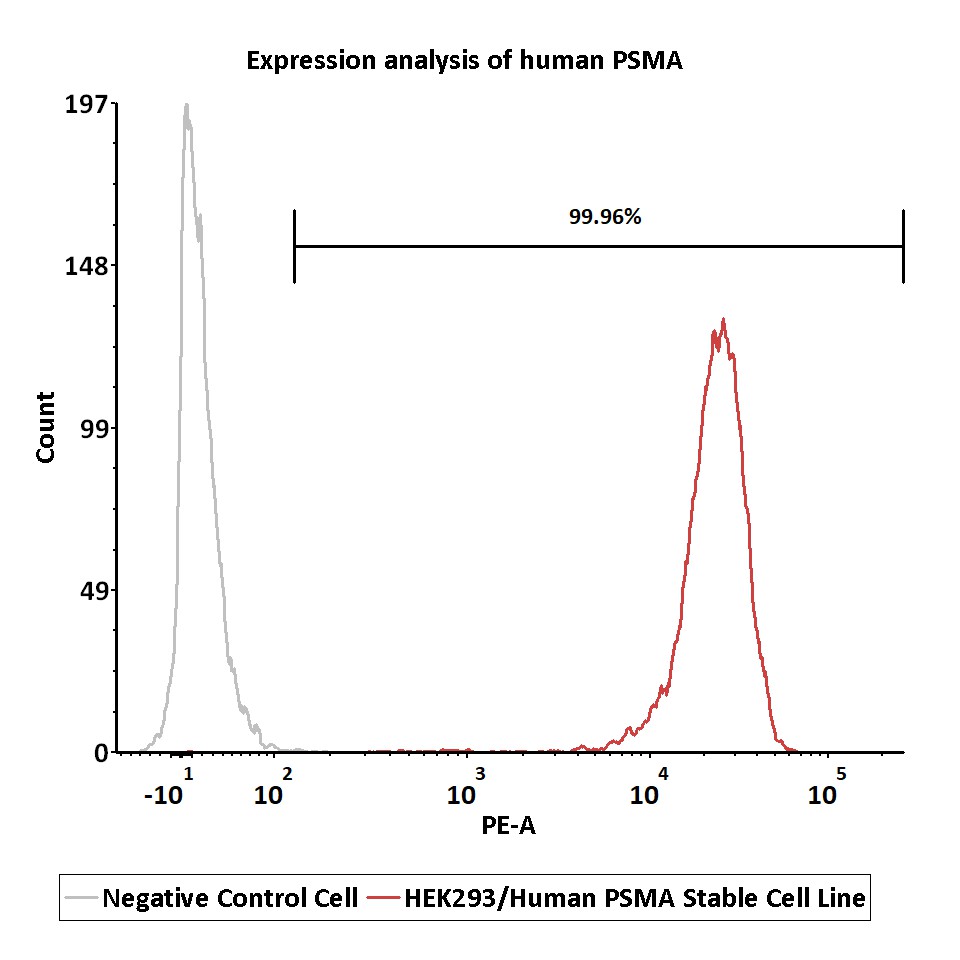
Expression analysis of human PSMA on HEK293/Human PSMA Stable Cell Line by FACS.
Cell surface staining was performed on HEK293/Human PSMA Stable Cell Line or negative control cell using PE anti-human PSMA antibody after fixation with 4% paraformaldehyde.

5e5 of anti-PSMA CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human PSMA, His Tag (Cat. No. PSA-HP2Q3) and negative control protein respectively. PE signal was used to evaluate the binding activity (QC tested).
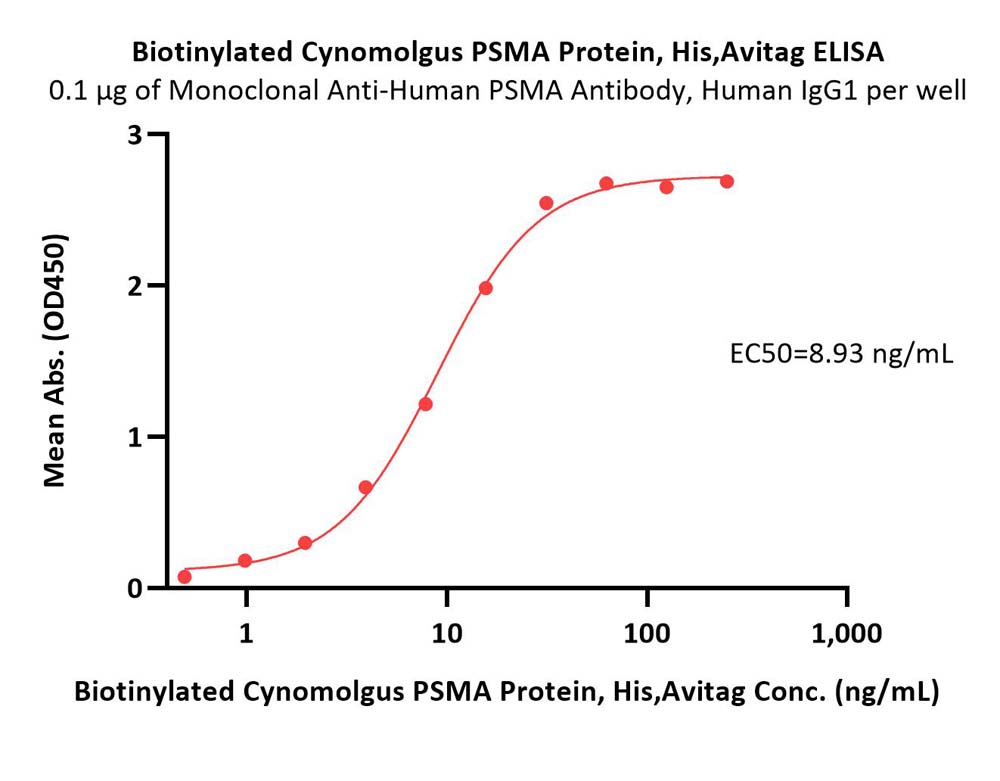
Immobilized Monoclonal Anti-Human PSMA Antibody, Human IgG1 at 1 μg/mL (100 μL/well) can bind Biotinylated Cynomolgus PSMA Protein, His,Avitag (Cat. No. PSA-C82Q6) with a linear range of 0.5-16 ng/mL (QC tested).
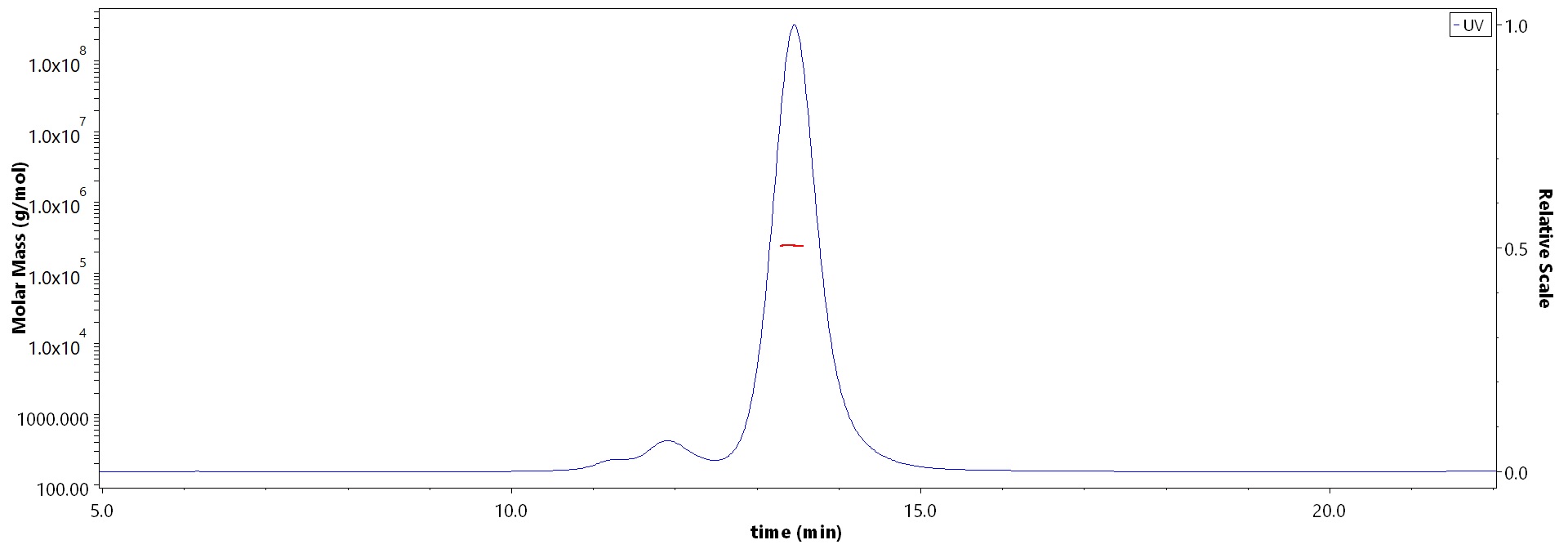
The purity of Biotinylated Mouse PSMA Protein, Fc,Avitag (Cat. No. PSA-M82F3) is more than 85% and the molecular weight of this protein is around 230-260 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Capromab pendetide | lndium-CYT-356; Indium-111-CYT-356; CYT-356; 111In CYT-356 | Approved | Eusa Pharma | ProstaScint | United States | Prostatic Neoplasms | Cytogen Corp | 1996-10-28 | Prostatic Neoplasms | Details |
| Piflufolastat F 18 | Approved | Johns Hopkins University | Pylarify, Pylclari | United States | Prostatic Neoplasms | Progenics Pharmaceuticals Inc | 2021-05-26 | Carcinoma, Renal Cell; Pancreatic Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Genital Neoplasms, Female; Diagnostic agents; Adenocarcinoma; Carcinoma, Hepatocellular; Neoplasm Metastasis | Details | |
| Ga-68 PSMA-11 | Ga-68-PSMA-11; Ga-68-PSMA; 68Ga-HBED-CC-PSMA11; AAA517; AAA-517 | Approved | Radiomedix Inc | Gallium Ga 68 Psma-11, Illuccix, ILLUCCIX, Locametz | United States | Prostatic Neoplasms | Telix Pharmaceuticals Ltd, University Of California, Los Angeles | 2020-12-01 | Neoplasm Recurrence, Local; Solid tumours; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Diagnostic agents; Neoplasm Metastasis; Carcinoma, Hepatocellular | Details |
| Lutetium (177Lu) vipivotide tetraxetan | Lutetium-177-PSMA-617; PSMA-617-[177Lu]; 177-Lu-PSMA-617; AAA-617; Lu-177- RLT | Approved | Radiomedix Inc | PLUVICTO | United States | Prostatic Neoplasms, Castration-Resistant | Novartis Pharmaceuticals Corp | 2022-03-23 | Carcinoma, Verrucous; Prostatic Neoplasms, Castration-Resistant; Carcinoma, Adenoid Cystic; Prostatic Neoplasms; Carcinoma, Squamous Cell; Neoplasm Metastasis | Details |
| Flotufolastat F-18 | Fluorine-18 rhPSMA; rhPSMA-7.3 (18F); 18F-rhPSMA-7.3; (18F)-rhPSMA-7.3; 18FrhPSMA-7.3; F-18-rhPSMA-7.3 | Approved | Blue Earth Diagnostics Inc, Technical University Munich | POSLUMA | United States | Prostatic Neoplasms | Blue Earth Diagnostics Ltd | 2023-05-25 | Prostatic Neoplasms; Contrast agents | Details |
| Capromab pendetide | lndium-CYT-356; Indium-111-CYT-356; CYT-356; 111In CYT-356 | Approved | Eusa Pharma | ProstaScint | United States | Prostatic Neoplasms | Cytogen Corp | 1996-10-28 | Prostatic Neoplasms | Details |
| Piflufolastat F 18 | Approved | Johns Hopkins University | Pylarify, Pylclari | United States | Prostatic Neoplasms | Progenics Pharmaceuticals Inc | 2021-05-26 | Carcinoma, Renal Cell; Pancreatic Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Genital Neoplasms, Female; Diagnostic agents; Adenocarcinoma; Carcinoma, Hepatocellular; Neoplasm Metastasis | Details | |
| Ga-68 PSMA-11 | Ga-68-PSMA-11; Ga-68-PSMA; 68Ga-HBED-CC-PSMA11; AAA517; AAA-517 | Approved | Radiomedix Inc | Gallium Ga 68 Psma-11, Illuccix, ILLUCCIX, Locametz | United States | Prostatic Neoplasms | Telix Pharmaceuticals Ltd, University Of California, Los Angeles | 2020-12-01 | Neoplasm Recurrence, Local; Solid tumours; Carcinoma, Renal Cell; Carcinoma, Ovarian Epithelial; Prostatic Neoplasms, Castration-Resistant; Urinary Bladder Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Diagnostic agents; Neoplasm Metastasis; Carcinoma, Hepatocellular | Details |
| Lutetium (177Lu) vipivotide tetraxetan | Lutetium-177-PSMA-617; PSMA-617-[177Lu]; 177-Lu-PSMA-617; AAA-617; Lu-177- RLT | Approved | Radiomedix Inc | PLUVICTO | United States | Prostatic Neoplasms, Castration-Resistant | Novartis Pharmaceuticals Corp | 2022-03-23 | Carcinoma, Verrucous; Prostatic Neoplasms, Castration-Resistant; Carcinoma, Adenoid Cystic; Prostatic Neoplasms; Carcinoma, Squamous Cell; Neoplasm Metastasis | Details |
| Flotufolastat F-18 | Fluorine-18 rhPSMA; rhPSMA-7.3 (18F); 18F-rhPSMA-7.3; (18F)-rhPSMA-7.3; 18FrhPSMA-7.3; F-18-rhPSMA-7.3 | Approved | Blue Earth Diagnostics Inc, Technical University Munich | POSLUMA | United States | Prostatic Neoplasms | Blue Earth Diagnostics Ltd | 2023-05-25 | Prostatic Neoplasms; Contrast agents | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| 18F-PSMA-1007 | 18F-PSMA-1007 | Phase 3 Clinical | Deutsches Krebsforschungszentrum | Neoplasms; Prostatic Neoplasms | Details |
| Gallium-68 PSMA-617 | 225 Ac-PSMA-617; 68Ga-PSMA-617; 68Ga-DKFZ-617; 68Ga-DKFZ-PSMA -617; Gallium-68-PSMA-617; PSMA-617-[68Ga]; 68Ga-DOTA-PSMA-DKFZ -617 | Phase 3 Clinical | Peking Union Medical College Hospital | Carcinoma, Adenoid Cystic; Prostatic Neoplasms | Details |
| Fluorine-18-CTT-1057 | CTT-1057; 18F-CTT1057; CTT-1057-18F; CTT-1057-F-18 | Phase 3 Clinical | Washington University, Cancer Targeted Technology Llc | Recurrence; Carcinoma, Renal Cell; Prostatic Neoplasms | Details |
| ITM-24D | ITM-24D; 68Ga-PSMA-TTM | Phase 3 Clinical | ITM Isotope Technologies Munich SE | Prostatic Neoplasms | Details |
| 18F vipivotide tetraxetan | 18F-PSMA-617; Al18F-PSMA-617 | Phase 3 Clinical | Peking Union Medical College Hospital | Prostatic Neoplasms | Details |
| 64Cu-PSMA I&T | Copper Cu 64 PSMA I&T; Cu-64-PSMA-I&T; 64-Copper-PSMA-I&T; Copper-64-prostate specific membrane antigen I&T | Phase 3 Clinical | Curium Us Llc | Prostatic Neoplasms | Details |
| Thretide[18F] | Phase 3 Clinical | Shanghai Lannacheng Biotechnology Co Ltd | Neoplasms; Prostatic Neoplasms; Diagnostic agents | Details | |
| 64Cu-SAR-bisPSMA (Clarity Pharmaceuticals) | Phase 3 Clinical | Clarity Pharmaceuticals Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| 177Lu-DOTA-rosopatamb | 177Lu-DOTA-TLX591-CHO; TLX591 | Phase 3 Clinical | Telix Pharmaceuticals Ltd | Prostatic Neoplasms | Details |
| [Lu177]-PNT-2002 | PNT-2002 | Phase 3 Clinical | Carcinoma, Adenoid Cystic; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| Lutetium-177-PSMA-I&T | Phase 2 Clinical | Radboud University Nijmegen | Salivary Gland Neoplasms; Carcinoma, Adenoid Cystic; Prostatic Neoplasms | Details | |
| 68Ga-PSMA-R2 (Advanced Accelerator Applications SA) | Phase 2 Clinical | Advanced Accelerator Applications Sa | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| 177Lu-PSMA-R2 | 177Lu-PSMA-R2; 177Lu-PSMA-SR6; AAA-602 | Phase 2 Clinical | Advanced Accelerator Applications Sa | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| 89Zr-DFO-huJ591 | Zr89-J591; 89Zr-DFO-J591; 89Zr-DFO-huJ591 | Phase 2 Clinical | Memorial Sloan Kettering Cancer Center | Prostatic Neoplasms | Details |
| Nezastomig | REGN-5678 | Phase 2 Clinical | Carcinoma, Renal Cell; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| 225Ac-J591 | 225Ac-DOTA-J591; 225Ac-J591; CONV 01-α | Phase 2 Clinical | Cornell University | Prostatic Neoplasms | Details |
| ATL-101 | MLN-591RL; ATL-101; huJ-591; MLN-591; J-591; Lu-177-J591; 177Lu-J591; 90Y-J591; muJ-591 | Phase 2 Clinical | Weill Medical College of Cornell University | Ovarian Neoplasms; Head and Neck Neoplasms; Kidney Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Glioma; Carcinoma, Non-Small-Cell Lung | Details |
| [131I]MIP-1095 | 1095; [131I]MIP-1095; [131I]MIP-1466 | Phase 2 Clinical | Progenics Pharmaceuticals Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| CONV-01-alpha + PSMA I&T | Phase 2 Clinical | Convergent Therapeutics Inc | Prostatic Neoplasms | Details | |
| LAVA-1207 | LAVA-1207 | Phase 2 Clinical | Vu University Medical Center, Lava Therapeutics NV | Prostatic Neoplasms, Castration-Resistant | Details |
| 4SCAR-PSMA T cell therapy (Shenzhen Geno-Immune Medical Institute) | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Neoplasms | Details | |
| [225Ac]Ac-PSMA-R2 | [225Ac]Ac-PSMA-R2 | Phase 2 Clinical | Novartis Pharma Ag | Prostatic Neoplasms, Castration-Resistant | Details |
| 177Lu-PSMA | 177Lu-PSMA | Phase 2 Clinical | Istituto Scientifico Romagnolo Per Lo Studio E La Cura Dei Tumori | Carcinoma, Renal Cell; Neoplasms; Prostatic Neoplasms; Neoplasm Metastasis | Details |
| [177Lu]JH020002 | Lu 177 JH020002; [177Lu]JH020002; 177-Lu JH-020002 | Phase 2 Clinical | Bivision Biomedical Technology (Nanjing) Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| [177Lu]Lu-XT033 | [177Lu]Lu-XT033 | Phase 2 Clinical | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| 177Lu-HTK03170 | 177Lu-HTK03170 | Phase 2 Clinical | British Columbia Cancer Agency | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| Ga-68-NGUL | Phase 2 Clinical | Cellbion Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| CD19/70 Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| GD2/PSMA Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR GD2/PSMA | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Solid tumours | Details |
| PSMA/CD70 Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR PSMA/CD70 | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Neoplasms | Details |
| 177Lu-rhPSMA-10.1 | 177Lu-rhPSMA10.1; (177Lu) rhPSMA-10.1; Lutetium (177Lu) rhPSMA-10.1 (Tx IMP); Lutetium-177 rhPSMA10.1; Lutetium Lu 177 PSMA-10.1; 177Lu rhPSMA-10.1; Lu177-rhPSMA; 177Lu Radiohybrid PSMA-10.1; 177Lu-rhPSMA-10.1; 177Lu-rhPSMA | Phase 2 Clinical | Technical University Munich | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Urogenital Neoplasms; Prostatic Diseases | Details |
| Lu-177-DGUL | PSMA-D GUL; Lu-177-DGUL; 177Lu-DOTA-GUL | Phase 2 Clinical | Cellbion Co Ltd | Neoplasms; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| TLX-592 | TLX-592 | Phase 2 Clinical | Telix Pharmaceuticals Ltd | Prostatic Neoplasms | Details |
| 68Ga-PSMA-IRDye | TLX591-Sx | Phase 2 Clinical | Telix Pharmaceuticals Ltd | Prostatic Neoplasms | Details |
| REGN-4336 | REGN-4336 | Phase 2 Clinical | Prostatic Neoplasms, Castration-Resistant | Details | |
| [177Lu]Ludotadipep | 177Lu-FC705; 177Lu-FC-705 | Phase 2 Clinical | Futurechem | Prostatic Neoplasms, Castration-Resistant | Details |
| 67Cu-SAR-bisPSMA (Clarity Pharmaceuticals) | Phase 2 Clinical | Clarity Pharmaceuticals Ltd | Prostatic Neoplasms, Castration-Resistant | Details | |
| ARX-517 | ARX-517; ARX517 | Phase 2 Clinical | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| OTL-78 | OTL-78 | Phase 2 Clinical | Purdue University, Novartis Pharma Ag | Prostatic Neoplasms | Details |
| INO-5401 | INO-5401 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | Carcinoma, Transitional Cell; Glioblastoma | Details |
| 225 Actinium PSMA-617 | 225 Actinium PSMA-617; 225Ac-PSMA 617; AAA 817; 225Ac-PSMA-617 | Phase 1 Clinical | Novartis Pharma Ag, Endocyte Inc, Advanced Accelerator Applications Sa | Prostatic Neoplasms, Castration-Resistant | Details |
| JNJ-63898081 | JNJ-8081; JNJ-63898081 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| P28z-CAR | P28z-CAR | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center, United States Department Of Defense | Prostatic Neoplasms | Details |
| PSMA-CART cell therapy (Shanghai Bioray Laboratory) | Phase 1 Clinical | BRL Medicine Inc | Prostatic Neoplasms, Castration-Resistant | Details | |
| Autologous T-cell therapy (anti-PSMA-CD3), Roger Williams Medical Center | Phase 1 Clinical | Roger Williams Medical Center | Prostatic Neoplasms | Details | |
| CC-1 | CC-1 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Squamous Cell | Details |
| Acapatamab | AMG-160 | Phase 1 Clinical | Amgen Inc | Prostatic Neoplasms, Castration-Resistant; Carcinoma, Non-Small-Cell Lung | Details |
| 177Lu-EB-vipivotide tetraxetan | 177-Lu-EB-PSMA-617 | Phase 1 Clinical | Peking Union Medical College Hospital, National Institute For Biomedical Imaging And Bioengineering (Nibib) | Carcinoma, Renal Cell; Carcinoma, Adenoid Cystic; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| [177Lu]-CTT-1403 | CTT-1403; [177Lu]CTT1403; 177Lutetium CTT1403; [177Lu]CTT-1403 | Phase 1 Clinical | Cancer Targeted Technology Llc | Prostatic Neoplasms | Details |
| Pelgifatamab corixetan | BAY-2315497 | Phase 1 Clinical | Bayer AG | Prostatic Neoplasms, Castration-Resistant | Details |
| 161Tb-SibuDAB | Phase 1 Clinical | University Hospital Basel | Prostatic Neoplasms, Castration-Resistant | Details | |
| [Ac-225]-PSMA-62 | [Ac-225]-PSMA-62 | Phase 1 Clinical | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| JNJ-9401 | JNJ-9401 | Phase 1 Clinical | Xencor Inc | Prostatic Neoplasms | Details |
| ABBV-969 | ABBV-969; ABBV969 | Phase 1 Clinical | Abbvie Inc, Immunogen Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| JNJ-87189401 | JNJ-87189401 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms | Details |
| Actinium-225-macropa-pelgifatamab | BAY-3546828; BAY3546828 | Phase 1 Clinical | Bayer AG | Prostatic Neoplasms, Castration-Resistant | Details |
| 177Lu-LNC1003 | 177Lu-LNC1003 | Phase 1 Clinical | Shanghai Lannacheng Biotechnology Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| PSMA-Targeted [In-111]-Labeled Trillium Compound | Phase 1 Clinical | Ratio Therapeutics Inc | Prostatic Neoplasms | Details | |
| Autologous T cells therapy(Unicar-Therapy) | Phase 1 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Prostatic Neoplasms | Details | |
| 212Pb-NG001 | 212Pb-NG001; AB-001; AB001 | Phase 1 Clinical | ARTBIO Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| CART-PSMA cells(Nova Therapeutics) | Phase 1 Clinical | Nova Therapeutics LLC | Prostatic Neoplasms | Details | |
| 68Ga-labeled NY108 | 68-Ga-labeled NY-108 | Phase 1 Clinical | Prostatic Neoplasms | Details | |
| JANX-007 | JANX-007; PSMA-TRACTr | Phase 1 Clinical | Janux Therapeutics Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| JNJ-80038114 | JNJ-80038114 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms | Details |
| CART-PSMA-TGFbRDN (University of Pennsylvania) | Phase 1 Clinical | University Of Pennsylvania | Prostatic Neoplasms | Details | |
| TNB-585 | TNB-585; AMG-340 | Phase 1 Clinical | Teneobio Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| AVC-102 | AVC-102 | Phase 1 Clinical | AvenCell Therapeutics Inc | Kidney Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| CBP-1018 | CBP-1018 | Phase 1 Clinical | Solid tumours; Carcinoma, Renal Cell; Prostatic Neoplasms; Lung Neoplasms | Details | |
| CCW-702 | CCW-702 | Phase 1 Clinical | The Scripps Research Institute Inc, Abbvie Inc | Prostatic Neoplasms | Details |
| Anti-PSMA CAR T-cell therapy (TNK Therapeutics) | Phase 1 Clinical | Sorrento Therapeutics Inc | Neoplasms | Details | |
| CART-PSMA-TGFβRDN cell therapy (Tmunity Therapeutics) | TmPSMA-02 | Phase 1 Clinical | University Of Minnesota, University Of Pennsylvania | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| 177Lu-PSMA-0057 | 177Lu-PSMA-0057 | Nanjing First Hospital, Nanjing Medical University | Details | ||
| [68Ga]Ga-PSMA-D5 | [68Ga]Ga-PSMA-D5 | Anhui Provincial Hospital | Details | ||
| 18F-PSMA-1007 | 18F-PSMA-1007 | Phase 3 Clinical | Deutsches Krebsforschungszentrum | Neoplasms; Prostatic Neoplasms | Details |
| Gallium-68 PSMA-617 | 225 Ac-PSMA-617; 68Ga-PSMA-617; 68Ga-DKFZ-617; 68Ga-DKFZ-PSMA -617; Gallium-68-PSMA-617; PSMA-617-[68Ga]; 68Ga-DOTA-PSMA-DKFZ -617 | Phase 3 Clinical | Peking Union Medical College Hospital | Carcinoma, Adenoid Cystic; Prostatic Neoplasms | Details |
| Fluorine-18-CTT-1057 | CTT-1057; 18F-CTT1057; CTT-1057-18F; CTT-1057-F-18 | Phase 3 Clinical | Washington University, Cancer Targeted Technology Llc | Recurrence; Carcinoma, Renal Cell; Prostatic Neoplasms | Details |
| ITM-24D | ITM-24D; 68Ga-PSMA-TTM | Phase 3 Clinical | ITM Isotope Technologies Munich SE | Prostatic Neoplasms | Details |
| 18F vipivotide tetraxetan | 18F-PSMA-617; Al18F-PSMA-617 | Phase 3 Clinical | Peking Union Medical College Hospital | Prostatic Neoplasms | Details |
| 64Cu-PSMA I&T | Copper Cu 64 PSMA I&T; Cu-64-PSMA-I&T; 64-Copper-PSMA-I&T; Copper-64-prostate specific membrane antigen I&T | Phase 3 Clinical | Curium Us Llc | Prostatic Neoplasms | Details |
| Thretide[18F] | Phase 3 Clinical | Shanghai Lannacheng Biotechnology Co Ltd | Neoplasms; Prostatic Neoplasms; Diagnostic agents | Details | |
| 64Cu-SAR-bisPSMA (Clarity Pharmaceuticals) | Phase 3 Clinical | Clarity Pharmaceuticals Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| 177Lu-DOTA-rosopatamb | 177Lu-DOTA-TLX591-CHO; TLX591 | Phase 3 Clinical | Telix Pharmaceuticals Ltd | Prostatic Neoplasms | Details |
| [Lu177]-PNT-2002 | PNT-2002 | Phase 3 Clinical | Carcinoma, Adenoid Cystic; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| Lutetium-177-PSMA-I&T | Phase 2 Clinical | Radboud University Nijmegen | Salivary Gland Neoplasms; Carcinoma, Adenoid Cystic; Prostatic Neoplasms | Details | |
| 68Ga-PSMA-R2 (Advanced Accelerator Applications SA) | Phase 2 Clinical | Advanced Accelerator Applications Sa | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| 177Lu-PSMA-R2 | 177Lu-PSMA-R2; 177Lu-PSMA-SR6; AAA-602 | Phase 2 Clinical | Advanced Accelerator Applications Sa | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| 89Zr-DFO-huJ591 | Zr89-J591; 89Zr-DFO-J591; 89Zr-DFO-huJ591 | Phase 2 Clinical | Memorial Sloan Kettering Cancer Center | Prostatic Neoplasms | Details |
| Nezastomig | REGN-5678 | Phase 2 Clinical | Carcinoma, Renal Cell; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| 225Ac-J591 | 225Ac-DOTA-J591; 225Ac-J591; CONV 01-α | Phase 2 Clinical | Cornell University | Prostatic Neoplasms | Details |
| ATL-101 | MLN-591RL; ATL-101; huJ-591; MLN-591; J-591; Lu-177-J591; 177Lu-J591; 90Y-J591; muJ-591 | Phase 2 Clinical | Weill Medical College of Cornell University | Ovarian Neoplasms; Head and Neck Neoplasms; Kidney Neoplasms; Esophageal Neoplasms; Pancreatic Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Glioma; Carcinoma, Non-Small-Cell Lung | Details |
| [131I]MIP-1095 | 1095; [131I]MIP-1095; [131I]MIP-1466 | Phase 2 Clinical | Progenics Pharmaceuticals Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| CONV-01-alpha + PSMA I&T | Phase 2 Clinical | Convergent Therapeutics Inc | Prostatic Neoplasms | Details | |
| LAVA-1207 | LAVA-1207 | Phase 2 Clinical | Vu University Medical Center, Lava Therapeutics NV | Prostatic Neoplasms, Castration-Resistant | Details |
| 4SCAR-PSMA T cell therapy (Shenzhen Geno-Immune Medical Institute) | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Neoplasms | Details | |
| [225Ac]Ac-PSMA-R2 | [225Ac]Ac-PSMA-R2 | Phase 2 Clinical | Novartis Pharma Ag | Prostatic Neoplasms, Castration-Resistant | Details |
| 177Lu-PSMA | 177Lu-PSMA | Phase 2 Clinical | Istituto Scientifico Romagnolo Per Lo Studio E La Cura Dei Tumori | Carcinoma, Renal Cell; Neoplasms; Prostatic Neoplasms; Neoplasm Metastasis | Details |
| [177Lu]JH020002 | Lu 177 JH020002; [177Lu]JH020002; 177-Lu JH-020002 | Phase 2 Clinical | Bivision Biomedical Technology (Nanjing) Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| [177Lu]Lu-XT033 | [177Lu]Lu-XT033 | Phase 2 Clinical | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| 177Lu-HTK03170 | 177Lu-HTK03170 | Phase 2 Clinical | British Columbia Cancer Agency | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| Ga-68-NGUL | Phase 2 Clinical | Cellbion Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| CD19/70 Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| GD2/PSMA Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR GD2/PSMA | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Solid tumours | Details |
| PSMA/CD70 Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR PSMA/CD70 | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Neoplasms | Details |
| 177Lu-rhPSMA-10.1 | 177Lu-rhPSMA10.1; (177Lu) rhPSMA-10.1; Lutetium (177Lu) rhPSMA-10.1 (Tx IMP); Lutetium-177 rhPSMA10.1; Lutetium Lu 177 PSMA-10.1; 177Lu rhPSMA-10.1; Lu177-rhPSMA; 177Lu Radiohybrid PSMA-10.1; 177Lu-rhPSMA-10.1; 177Lu-rhPSMA | Phase 2 Clinical | Technical University Munich | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Urogenital Neoplasms; Prostatic Diseases | Details |
| Lu-177-DGUL | PSMA-D GUL; Lu-177-DGUL; 177Lu-DOTA-GUL | Phase 2 Clinical | Cellbion Co Ltd | Neoplasms; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| TLX-592 | TLX-592 | Phase 2 Clinical | Telix Pharmaceuticals Ltd | Prostatic Neoplasms | Details |
| 68Ga-PSMA-IRDye | TLX591-Sx | Phase 2 Clinical | Telix Pharmaceuticals Ltd | Prostatic Neoplasms | Details |
| REGN-4336 | REGN-4336 | Phase 2 Clinical | Prostatic Neoplasms, Castration-Resistant | Details | |
| [177Lu]Ludotadipep | 177Lu-FC705; 177Lu-FC-705 | Phase 2 Clinical | Futurechem | Prostatic Neoplasms, Castration-Resistant | Details |
| 67Cu-SAR-bisPSMA (Clarity Pharmaceuticals) | Phase 2 Clinical | Clarity Pharmaceuticals Ltd | Prostatic Neoplasms, Castration-Resistant | Details | |
| ARX-517 | ARX-517; ARX517 | Phase 2 Clinical | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| OTL-78 | OTL-78 | Phase 2 Clinical | Purdue University, Novartis Pharma Ag | Prostatic Neoplasms | Details |
| INO-5401 | INO-5401 | Phase 2 Clinical | Inovio Pharmaceuticals Inc | Carcinoma, Transitional Cell; Glioblastoma | Details |
| 225 Actinium PSMA-617 | 225 Actinium PSMA-617; 225Ac-PSMA 617; AAA 817; 225Ac-PSMA-617 | Phase 1 Clinical | Novartis Pharma Ag, Endocyte Inc, Advanced Accelerator Applications Sa | Prostatic Neoplasms, Castration-Resistant | Details |
| JNJ-63898081 | JNJ-8081; JNJ-63898081 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Neoplasms | Details |
| P28z-CAR | P28z-CAR | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center, United States Department Of Defense | Prostatic Neoplasms | Details |
| PSMA-CART cell therapy (Shanghai Bioray Laboratory) | Phase 1 Clinical | BRL Medicine Inc | Prostatic Neoplasms, Castration-Resistant | Details | |
| Autologous T-cell therapy (anti-PSMA-CD3), Roger Williams Medical Center | Phase 1 Clinical | Roger Williams Medical Center | Prostatic Neoplasms | Details | |
| CC-1 | CC-1 | Phase 1 Clinical | University Of Tubingen, German Cancer Research Center (Dkfz) | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms; Carcinoma, Squamous Cell | Details |
| Acapatamab | AMG-160 | Phase 1 Clinical | Amgen Inc | Prostatic Neoplasms, Castration-Resistant; Carcinoma, Non-Small-Cell Lung | Details |
| 177Lu-EB-vipivotide tetraxetan | 177-Lu-EB-PSMA-617 | Phase 1 Clinical | Peking Union Medical College Hospital, National Institute For Biomedical Imaging And Bioengineering (Nibib) | Carcinoma, Renal Cell; Carcinoma, Adenoid Cystic; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| [177Lu]-CTT-1403 | CTT-1403; [177Lu]CTT1403; 177Lutetium CTT1403; [177Lu]CTT-1403 | Phase 1 Clinical | Cancer Targeted Technology Llc | Prostatic Neoplasms | Details |
| Pelgifatamab corixetan | BAY-2315497 | Phase 1 Clinical | Bayer AG | Prostatic Neoplasms, Castration-Resistant | Details |
| 161Tb-SibuDAB | Phase 1 Clinical | University Hospital Basel | Prostatic Neoplasms, Castration-Resistant | Details | |
| [Ac-225]-PSMA-62 | [Ac-225]-PSMA-62 | Phase 1 Clinical | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| JNJ-9401 | JNJ-9401 | Phase 1 Clinical | Xencor Inc | Prostatic Neoplasms | Details |
| ABBV-969 | ABBV-969; ABBV969 | Phase 1 Clinical | Abbvie Inc, Immunogen Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| JNJ-87189401 | JNJ-87189401 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms | Details |
| Actinium-225-macropa-pelgifatamab | BAY-3546828; BAY3546828 | Phase 1 Clinical | Bayer AG | Prostatic Neoplasms, Castration-Resistant | Details |
| 177Lu-LNC1003 | 177Lu-LNC1003 | Phase 1 Clinical | Shanghai Lannacheng Biotechnology Co Ltd | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| PSMA-Targeted [In-111]-Labeled Trillium Compound | Phase 1 Clinical | Ratio Therapeutics Inc | Prostatic Neoplasms | Details | |
| Autologous T cells therapy(Unicar-Therapy) | Phase 1 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Prostatic Neoplasms | Details | |
| 212Pb-NG001 | 212Pb-NG001; AB-001; AB001 | Phase 1 Clinical | ARTBIO Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| CART-PSMA cells(Nova Therapeutics) | Phase 1 Clinical | Nova Therapeutics LLC | Prostatic Neoplasms | Details | |
| 68Ga-labeled NY108 | 68-Ga-labeled NY-108 | Phase 1 Clinical | Prostatic Neoplasms | Details | |
| JANX-007 | JANX-007; PSMA-TRACTr | Phase 1 Clinical | Janux Therapeutics Inc | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| JNJ-80038114 | JNJ-80038114 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms | Details |
| CART-PSMA-TGFbRDN (University of Pennsylvania) | Phase 1 Clinical | University Of Pennsylvania | Prostatic Neoplasms | Details | |
| TNB-585 | TNB-585; AMG-340 | Phase 1 Clinical | Teneobio Inc | Prostatic Neoplasms, Castration-Resistant | Details |
| AVC-102 | AVC-102 | Phase 1 Clinical | AvenCell Therapeutics Inc | Kidney Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| CBP-1018 | CBP-1018 | Phase 1 Clinical | Solid tumours; Carcinoma, Renal Cell; Prostatic Neoplasms; Lung Neoplasms | Details | |
| CCW-702 | CCW-702 | Phase 1 Clinical | The Scripps Research Institute Inc, Abbvie Inc | Prostatic Neoplasms | Details |
| Anti-PSMA CAR T-cell therapy (TNK Therapeutics) | Phase 1 Clinical | Sorrento Therapeutics Inc | Neoplasms | Details | |
| CART-PSMA-TGFβRDN cell therapy (Tmunity Therapeutics) | TmPSMA-02 | Phase 1 Clinical | University Of Minnesota, University Of Pennsylvania | Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details |
| 177Lu-PSMA-0057 | 177Lu-PSMA-0057 | Nanjing First Hospital, Nanjing Medical University | Details | ||
| [68Ga]Ga-PSMA-D5 | [68Ga]Ga-PSMA-D5 | Anhui Provincial Hospital | Details |
This web search service is supported by Google Inc.