 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
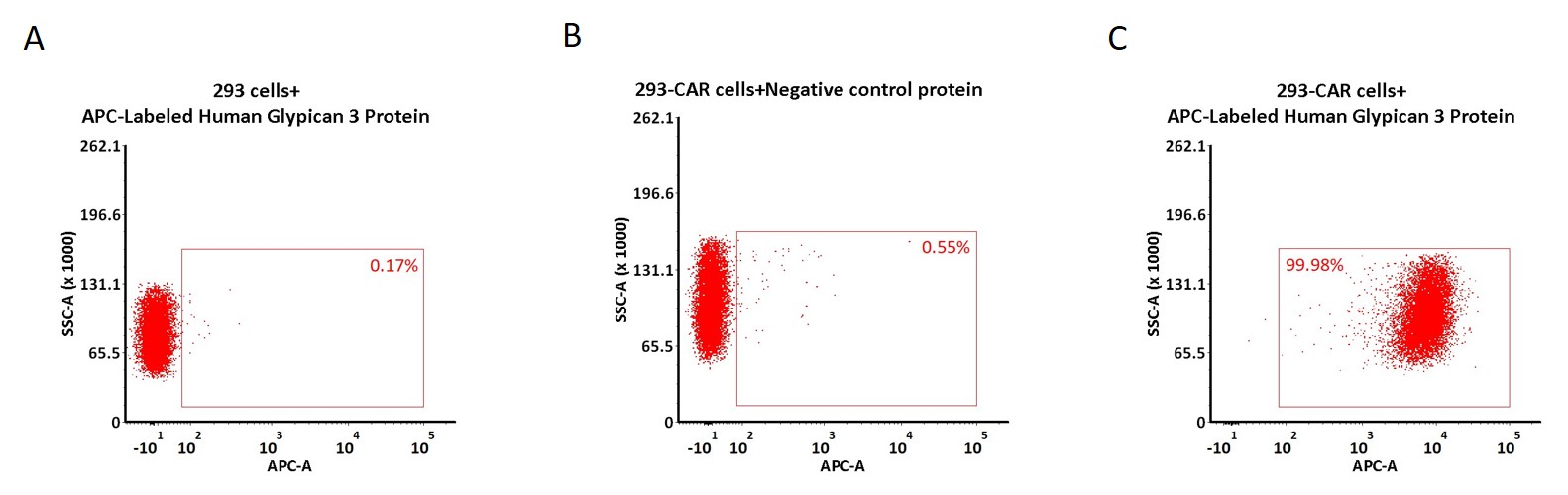
5e5 of anti-GPC3 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of APC-Labeled Human Glypican 3 Protein, His Tag (Cat. No. GP3-HA2H7) and negative control protein respectively (Fig. C and B), and non-transfected 293 cells were used as a control (Fig. A). APC signal was used to evaluate the binding activity (QC tested).
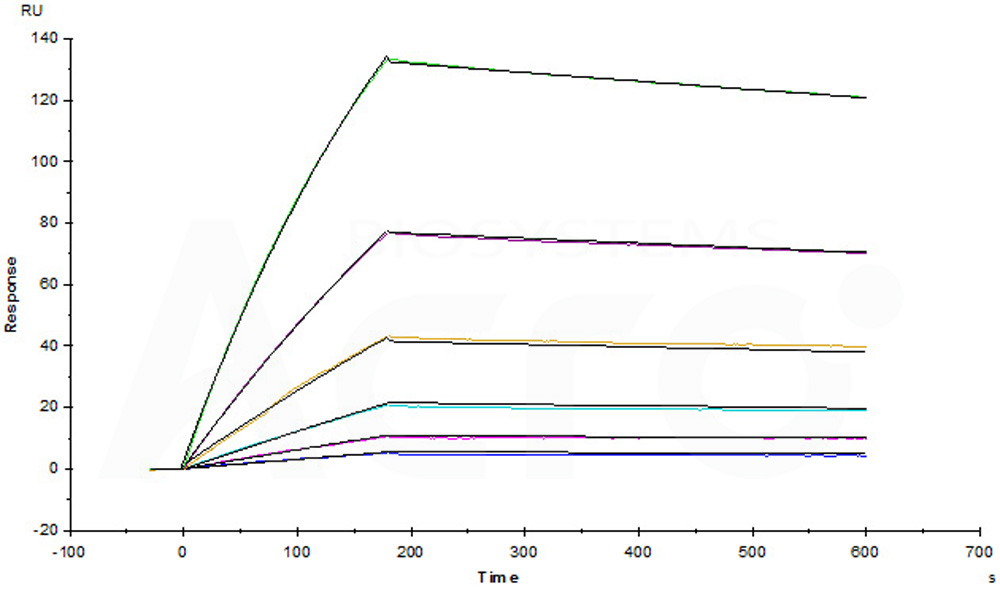
Anti-human GPC3 MAb (chimeric mouse-human IgG1) captured on CM5 chip via anti-human IgG Fc antibodies surface, can bind Human Glypican 3, His Tag, premium grade (Cat. No. GP3-H52H4) with an affinity constant of 1.33 nM as determined in a SPR assay (Biacore T200) (Routinely tested).

Anti-Human GPC3 MAb (chimeric mouse-human IgG1) captured on CM5 chip via anti-human IgG Fc antibodies surface, can bind Human Glypican 3 (S359F) Protein, His Tag (Cat. No. GP3-H5223) with an affinity constant of 1.96 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| GPC-3298306 | GPC-3298306 | Phase 2 Clinical | National Cancer Center Of Japan | Ovarian Neoplasms; Carcinoma, Hepatocellular | Details |
| BOS-342 | PRS-342; BOS-342; PRS-342/BOS-342 | Phase 2 Clinical | Pieris Pharmaceuticals | Solid tumours; Carcinoma, Hepatocellular | Details |
| BOXR-1030 | BOXR-1030 | Phase 2 Clinical | Unum Therapeutics Inc | Liver Neoplasms; Carcinoma, Merkel Cell; Carcinoma, Squamous Cell; Lung Neoplasms; Liposarcoma, Myxoid; Carcinoma, Hepatocellular | Details |
| AZD-5851 | AZD-5851; AZD5851 | Phase 2 Clinical | Astrazeneca Plc | Carcinoma, Hepatocellular | Details |
| SAR-444200 | SAR-444200 | Phase 2 Clinical | Sanofi | Solid tumours; Neoplasms | Details |
| CAR-GPC3 T-cell (Drum Tower Hospital) | Phase 2 Clinical | Nanjing Drum Tower Hospital | Carcinoma, Hepatocellular | Details | |
| GPC3 CAR-T therapy (Hrain Biotechnology) | Phase 2 Clinical | Hrain Biotechnology Co Ltd | Carcinoma, Hepatocellular | Details | |
| CM-350 | CM-350 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Solid tumours | Details |
| GPC3-CAR-T cell therapy(Origincell Medical Technology) | Ori-CAR-001; Ori-C101(OriCell) | Phase 2 Clinical | OriCell Therapeutics Co Ltd | Carcinoma, Hepatocellular | Details |
| ECT-204 | JWATM-204; ECT-204 | Phase 2 Clinical | Eureka Therapeutics Inc, Jw Therapeutics (Shanghai) Co Ltd | Liver Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Codrituzumab | GC-33; RG-7686; RO-5137382 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd, F. Hoffmann-La Roche Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| GPC3-T2 CAR-T cell therapy (The Second Affiliated Hospital of Guangzhou Medical University) | Phase 1 Clinical | Second Affiliated Hospital Of Guangzhou Medical University | Carcinoma, Squamous Cell; Carcinoma, Hepatocellular | Details | |
| GLYCAR T cell therapy (Baylor College of Medicine) | GLYCAR | Phase 1 Clinical | Baylor College Of Medicine | Rhabdomyosarcoma; Liver Neoplasms; Rhabdoid Tumor; Hepatoblastoma; Wilms Tumor; Liposarcoma; Carcinoma, Hepatocellular; Endodermal Sinus Tumor | Details |
| Anti-GPC3 CAR T-cell therapy (Nanjing University) | Phase 1 Clinical | Nanjing University | Carcinoma, Hepatocellular | Details | |
| GPC3-CAR-T cell therapy | CSG-GPC-3; CAR-GPC3 T-cell; GPC3-CAR/CSG-GPC3; anti-GPC-3 CAR T; KJgpc3-001; CT-011 | Phase 1 Clinical | Carsgen Biomedicine (Shanghai) Co Ltd | Solid tumours; Carcinoma, Squamous Cell; Carcinoma, Hepatocellular | Details |
| Anti-GPC3 chimeric antigen receptor T cell therapy (Hunan Zhaotai Yongren Biotech/Guangdong Zhaotai Invivo Biomedicine) | Phase 1 Clinical | Hunan Zhaotai Yongren Biotech Co Ltd, Guangdong Zhaotai Invivo Biomedicine Co Ltd | Carcinoma, Squamous Cell; Carcinoma, Hepatocellular | Details | |
| EU-307 | EU307 | Phase 1 Clinical | Eutilex | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| BC-2027 | BC2027; BC-2027 | Phase 1 Clinical | Biocity Biopharmaceutics Co Ltd | Liver Neoplasms; Solid tumours | Details |
| JMT-106 | JMT106; JMT-106 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd | Solid tumours | Details |
| GPC3/Mesothelin-CAR-γδT Cells therapy(Second Affiliated Hospital Of Guangzhou Medical University) | Phase 1 Clinical | Second Affiliated Hospital Of Guangzhou Medical University | Solid tumours; Liver Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Mesothelioma; Lung Neoplasms | Details | |
| NWRD-06 | NWRD-06; NWRD06 | Phase 1 Clinical | Newish Technology (Beijing) Co Ltd | Carcinoma, Hepatocellular | Details |
| GPC3-targeted CAR-T (Peking University) | Phase 1 Clinical | Peking University | Carcinoma, Hepatocellular | Details | |
| CAR (hYP7)-T cells (National Cancer Institute) | Phase 1 Clinical | National Cancer Institute | Liver Neoplasms; Carcinoma, Hepatocellular | Details | |
| CT0181 | CT-0181 | Phase 1 Clinical | CARsgen Therapeutics Holdings Ltd | Carcinoma, Hepatocellular | Details |
| TC-CAR-031 | C-CAR-031; TC-CAR-031; TC-CAR031; C-CAR031 | Phase 1 Clinical | Zhejiang University, Cellular Biomedicine Group Inc | Carcinoma, Hepatocellular | Details |
| IM-83 CAR-T cell therapy | IM-83; IM83 | Phase 1 Clinical | Beijing Immunochina Medical Science & Technology Co Ltd | Liver Neoplasms; Neoplasms; Carcinoma, Hepatocellular | Details |
| CT-017 | CT-017 | Phase 1 Clinical | Carsgen Biomedicine (Shanghai) Co Ltd | Carcinoma, Hepatocellular | Details |
| JWATM-214 | JWATM-214; JWATM214 | Phase 1 Clinical | Suzhou Yaomingjunuo Biotechnology Co Ltd | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| TH-012(Bangentai Biomedical) | TH-012 | Phase 1 Clinical | Shandong Bangentai Biomedical Tech Group Co Ltd | Stomach Neoplasms; Pancreatic Neoplasms | Details |
| B010-A | B010-A | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Carcinoma, Hepatocellular | Details |
| CT-0180 | CT-0180 | Phase 1 Clinical | CARsgen Therapeutics Holdings Ltd | Carcinoma, Hepatocellular | Details |
| ERY-974 | ERY-974 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| TAK-102 | TAK-102 | Phase 1 Clinical | Noile-Immune Biotech Inc | Solid tumours; Neoplasms | Details |
| C-CAR031 CAR T-cell therapy (AbelZeta) | C-CAR031 | Clinical | AbelZeta Pharma Inc | Carcinoma, Hepatocellular | Details |
| Anti-GPC3-IRDye800CW | Clinical | Chinese Academy Of Sciences | Details | ||
| GPC-3298306 | GPC-3298306 | Phase 2 Clinical | National Cancer Center Of Japan | Ovarian Neoplasms; Carcinoma, Hepatocellular | Details |
| BOS-342 | PRS-342; BOS-342; PRS-342/BOS-342 | Phase 2 Clinical | Pieris Pharmaceuticals | Solid tumours; Carcinoma, Hepatocellular | Details |
| BOXR-1030 | BOXR-1030 | Phase 2 Clinical | Unum Therapeutics Inc | Liver Neoplasms; Carcinoma, Merkel Cell; Carcinoma, Squamous Cell; Lung Neoplasms; Liposarcoma, Myxoid; Carcinoma, Hepatocellular | Details |
| AZD-5851 | AZD-5851; AZD5851 | Phase 2 Clinical | Astrazeneca Plc | Carcinoma, Hepatocellular | Details |
| SAR-444200 | SAR-444200 | Phase 2 Clinical | Sanofi | Solid tumours; Neoplasms | Details |
| CAR-GPC3 T-cell (Drum Tower Hospital) | Phase 2 Clinical | Nanjing Drum Tower Hospital | Carcinoma, Hepatocellular | Details | |
| GPC3 CAR-T therapy (Hrain Biotechnology) | Phase 2 Clinical | Hrain Biotechnology Co Ltd | Carcinoma, Hepatocellular | Details | |
| CM-350 | CM-350 | Phase 2 Clinical | Keymed Biosciences Co Ltd | Solid tumours | Details |
| GPC3-CAR-T cell therapy(Origincell Medical Technology) | Ori-CAR-001; Ori-C101(OriCell) | Phase 2 Clinical | OriCell Therapeutics Co Ltd | Carcinoma, Hepatocellular | Details |
| ECT-204 | JWATM-204; ECT-204 | Phase 2 Clinical | Eureka Therapeutics Inc, Jw Therapeutics (Shanghai) Co Ltd | Liver Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Codrituzumab | GC-33; RG-7686; RO-5137382 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd, F. Hoffmann-La Roche Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| GPC3-T2 CAR-T cell therapy (The Second Affiliated Hospital of Guangzhou Medical University) | Phase 1 Clinical | Second Affiliated Hospital Of Guangzhou Medical University | Carcinoma, Squamous Cell; Carcinoma, Hepatocellular | Details | |
| GLYCAR T cell therapy (Baylor College of Medicine) | GLYCAR | Phase 1 Clinical | Baylor College Of Medicine | Rhabdomyosarcoma; Liver Neoplasms; Rhabdoid Tumor; Hepatoblastoma; Wilms Tumor; Liposarcoma; Carcinoma, Hepatocellular; Endodermal Sinus Tumor | Details |
| Anti-GPC3 CAR T-cell therapy (Nanjing University) | Phase 1 Clinical | Nanjing University | Carcinoma, Hepatocellular | Details | |
| GPC3-CAR-T cell therapy | CSG-GPC-3; CAR-GPC3 T-cell; GPC3-CAR/CSG-GPC3; anti-GPC-3 CAR T; KJgpc3-001; CT-011 | Phase 1 Clinical | Carsgen Biomedicine (Shanghai) Co Ltd | Solid tumours; Carcinoma, Squamous Cell; Carcinoma, Hepatocellular | Details |
| Anti-GPC3 chimeric antigen receptor T cell therapy (Hunan Zhaotai Yongren Biotech/Guangdong Zhaotai Invivo Biomedicine) | Phase 1 Clinical | Hunan Zhaotai Yongren Biotech Co Ltd, Guangdong Zhaotai Invivo Biomedicine Co Ltd | Carcinoma, Squamous Cell; Carcinoma, Hepatocellular | Details | |
| EU-307 | EU307 | Phase 1 Clinical | Eutilex | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| BC-2027 | BC2027; BC-2027 | Phase 1 Clinical | Biocity Biopharmaceutics Co Ltd | Liver Neoplasms; Solid tumours | Details |
| JMT-106 | JMT106; JMT-106 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd | Solid tumours | Details |
| GPC3/Mesothelin-CAR-γδT Cells therapy(Second Affiliated Hospital Of Guangzhou Medical University) | Phase 1 Clinical | Second Affiliated Hospital Of Guangzhou Medical University | Solid tumours; Liver Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms; Mesothelioma; Lung Neoplasms | Details | |
| NWRD-06 | NWRD-06; NWRD06 | Phase 1 Clinical | Newish Technology (Beijing) Co Ltd | Carcinoma, Hepatocellular | Details |
| GPC3-targeted CAR-T (Peking University) | Phase 1 Clinical | Peking University | Carcinoma, Hepatocellular | Details | |
| CAR (hYP7)-T cells (National Cancer Institute) | Phase 1 Clinical | National Cancer Institute | Liver Neoplasms; Carcinoma, Hepatocellular | Details | |
| CT0181 | CT-0181 | Phase 1 Clinical | CARsgen Therapeutics Holdings Ltd | Carcinoma, Hepatocellular | Details |
| TC-CAR-031 | C-CAR-031; TC-CAR-031; TC-CAR031; C-CAR031 | Phase 1 Clinical | Zhejiang University, Cellular Biomedicine Group Inc | Carcinoma, Hepatocellular | Details |
| IM-83 CAR-T cell therapy | IM-83; IM83 | Phase 1 Clinical | Beijing Immunochina Medical Science & Technology Co Ltd | Liver Neoplasms; Neoplasms; Carcinoma, Hepatocellular | Details |
| CT-017 | CT-017 | Phase 1 Clinical | Carsgen Biomedicine (Shanghai) Co Ltd | Carcinoma, Hepatocellular | Details |
| JWATM-214 | JWATM-214; JWATM214 | Phase 1 Clinical | Suzhou Yaomingjunuo Biotechnology Co Ltd | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| TH-012(Bangentai Biomedical) | TH-012 | Phase 1 Clinical | Shandong Bangentai Biomedical Tech Group Co Ltd | Stomach Neoplasms; Pancreatic Neoplasms | Details |
| B010-A | B010-A | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Carcinoma, Hepatocellular | Details |
| CT-0180 | CT-0180 | Phase 1 Clinical | CARsgen Therapeutics Holdings Ltd | Carcinoma, Hepatocellular | Details |
| ERY-974 | ERY-974 | Phase 1 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours; Carcinoma, Hepatocellular | Details |
| TAK-102 | TAK-102 | Phase 1 Clinical | Noile-Immune Biotech Inc | Solid tumours; Neoplasms | Details |
| C-CAR031 CAR T-cell therapy (AbelZeta) | C-CAR031 | Clinical | AbelZeta Pharma Inc | Carcinoma, Hepatocellular | Details |
| Anti-GPC3-IRDye800CW | Clinical | Chinese Academy Of Sciences | Details |
This web search service is supported by Google Inc.