 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| PGF-H52G3 | Human | Human PlGF-1 Protein, GST Tag |  |
||
| PGF-H5255 | Human | Human PLGF-3 Protein, Fc Tag (MALS verified) |  |

|
|
| PGF-H5256 | Human | Human PlGF-1 Protein, Fc Tag (MALS verified) |  |

|
|
| PGF-H52H5 | Human | Human PlGF-1 Protein, His Tag (MALS verified) |  |
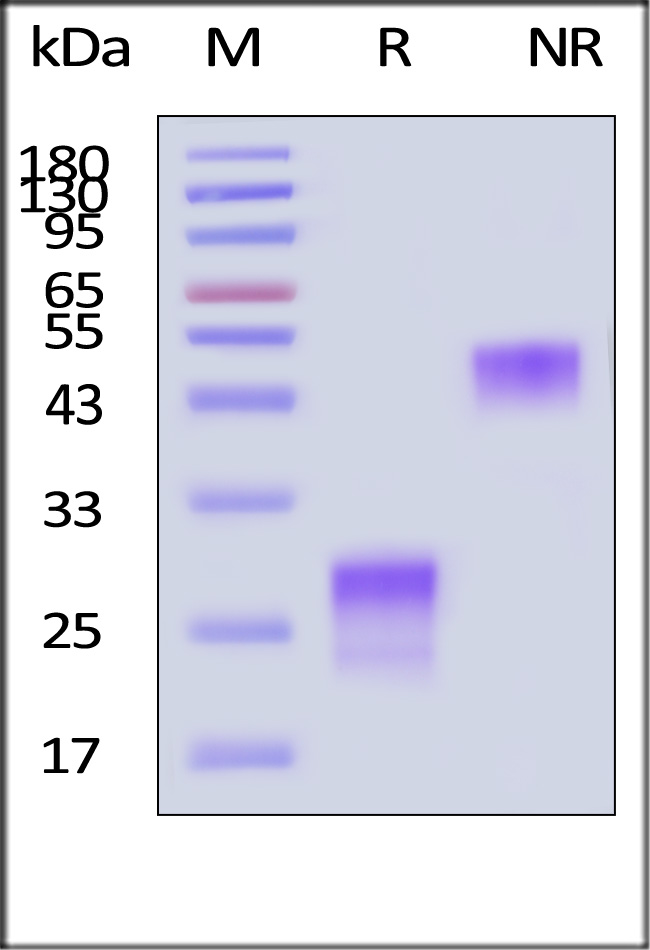
|
|
| PGF-M52H0 | Mouse | Mouse PLGF / PGF Protein, His Tag |  |
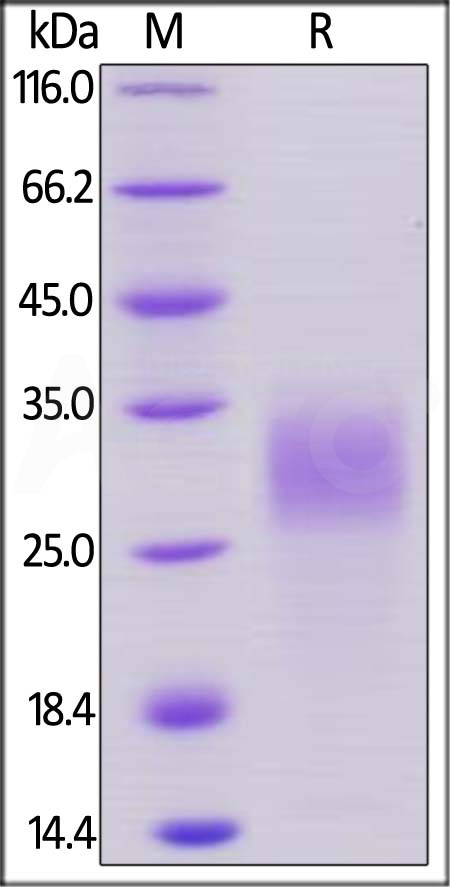
|
|
| PGF-R52H0 | Rhesus macaque | Rhesus macaque PLGF / PGF Protein, His Tag |  |
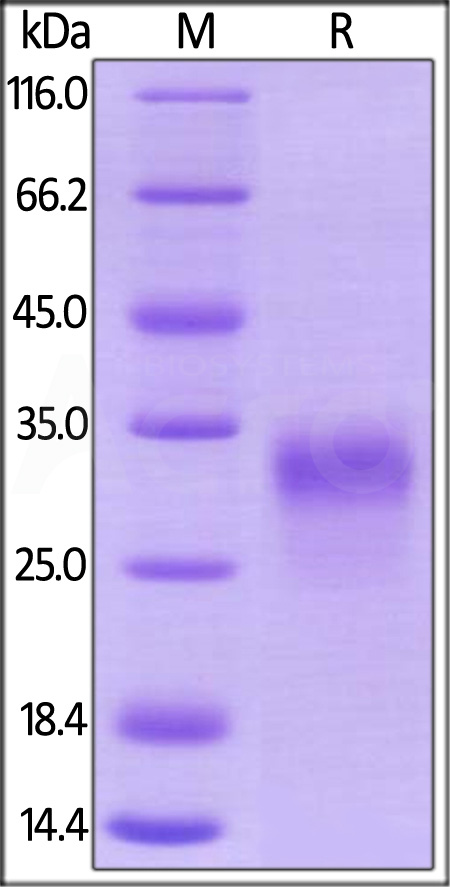
|
|
| PGF-H5229 | Human | Human PlGF-2 Protein, His Tag |  |

|
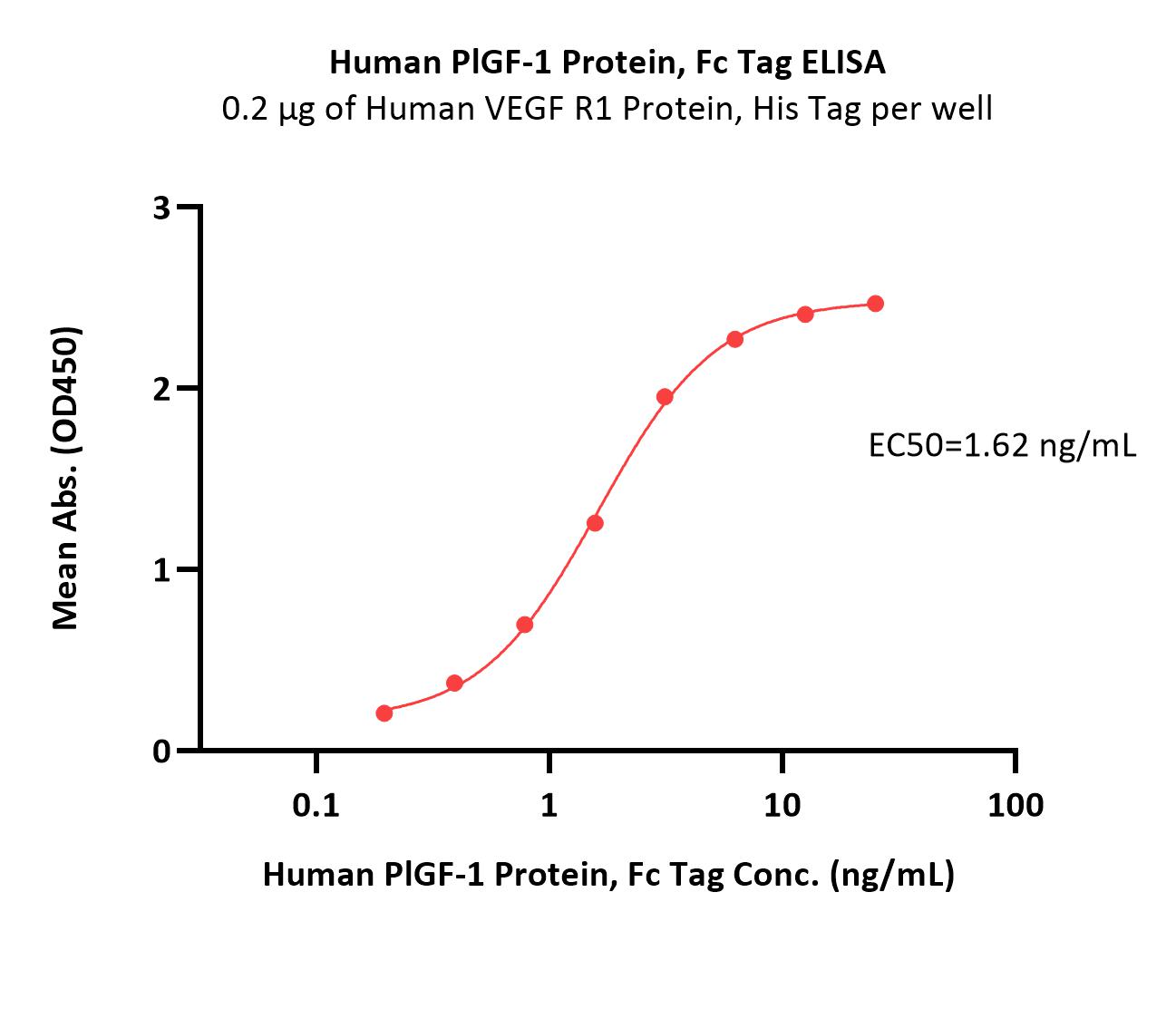
Immobilized Human VEGF R1 Protein, His Tag (Cat. No. VE1-H52H9) at 2 μg/mL (100 μL/well) can bind Human PlGF-1 Protein, Fc Tag (Cat. No. PGF-H5256) with a linear range of 0.1-3 ng/mL (QC tested).

The purity of Human PLGF-3 Protein, Fc Tag (Cat. No. PGF-H5255) is more than 85% and the molecular weight of this protein is around 115-130 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Aflibercept biosimilar (Momenta/Mylan) | M-710; MYL-1701P | Approved | Momenta, Mylan Nv | EU | Diabetes Complications; Myopia, Degenerative; Diabetic Retinopathy; Macular Edema; Retinal Vein Occlusion | Viatris Ltd | 2023-09-15 | Macular Edema; Myopia, Degenerative; Diabetic macular oedema; Diabetes Complications; Diabetic Retinopathy; Retinal Vein Occlusion | Details | |
| Aflibercept biosimilar (Qilu Pharmaceutical) | QL-1207 | Approved | Qilu Pharmaceutical Co Ltd | Mainland China | Macular Degeneration; Diabetic macular oedema | Qilu Pharmaceutical Co Ltd | 2023-12-13 | Diabetic macular oedema; Macular Degeneration | Details | |
| Aflibercept | BAY-865321; BAT-86-5321 | Approved | Bayer AG | Eylea, 艾力雅 | United States | Macular Degeneration; Macular Edema | Regeneron Pharmaceuticals Inc | 2011-11-18 | Retinitis Pigmentosa; Choroidal Neovascularization; Carcinoma, Non-Small-Cell Lung; Corneal Neovascularization; Retinopathy of Prematurity; Neoplasm Metastasis; Diabetic Retinopathy; Retinal Vein Occlusion; Macular Degeneration; Vitreous Hemorrhage; Lymphoma, Non-Hodgkin; Retinal Degeneration; Eye Diseases; Diabetes Complications; Colorectal Neoplasms; Prostatic Neoplasms; Diabetes Mellitus, Type 1; Wet Macular Degeneration; Diabetic macular oedema; Multiple Myeloma; Myopia, Degenerative; Retinal Diseases; Colonic Neoplasms; Central Serous Chorioretinopathy; Neoplasms; Diabetes Mellitus, Type 2; Rectal Neoplasms; Choroid Diseases; Glaucoma, Neovascular; Macular Edema; Cataract | Details |
| Ziv-aflibercept | BAY-865321; AVE-0005 | Approved | Sanofi | Zaltrap | United States | Colorectal Neoplasms | Sanofi-Aventis U.S. Llc | 2012-08-03 | Lymphoma; Breast Neoplasms; Urethral Neoplasms; Brain Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Carcinoma, Mucoepidermoid; Gliosarcoma; Astrocytoma; Leukemia, Myeloid, Chronic, Atypical, BCR-ABL Negative; Peritoneal Neoplasms; Ureteral Neoplasms; Lung Neoplasms; Prostatic Neoplasms; Uterine Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Neuroendocrine; Glioma; Lymphoma, Non-Hodgkin; Thyroid Neoplasms; Carcinoma, Squamous Cell; Retinal Vein Occlusion; Neoplasm Metastasis; Melanoma; Carcinoma, Non-Small-Cell Lung; Glioblastoma; Solid tumours; Leiomyosarcoma; Multiple Endocrine Neoplasia Type 1; Leukemia; Carcinoma; Carcinoma, Renal Cell; Carcinoid Tumor; Rectal Neoplasms; Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Leukemia, Myelomonocytic, Chronic; Ovarian Neoplasms; Myelodysplastic Syndromes; Myeloproliferative Disorders; Carcinoma, Transitional Cell; Carcinoma, Papillary; Small Cell Lung Carcinoma; Colonic Neoplasms; Ascites; Lung Diseases; Adenoc | Details |
| Aflibercept biosimilar (Momenta/Mylan) | M-710; MYL-1701P | Approved | Momenta, Mylan Nv | EU | Diabetes Complications; Myopia, Degenerative; Diabetic Retinopathy; Macular Edema; Retinal Vein Occlusion | Viatris Ltd | 2023-09-15 | Macular Edema; Myopia, Degenerative; Diabetic macular oedema; Diabetes Complications; Diabetic Retinopathy; Retinal Vein Occlusion | Details | |
| Aflibercept biosimilar (Qilu Pharmaceutical) | QL-1207 | Approved | Qilu Pharmaceutical Co Ltd | Mainland China | Macular Degeneration; Diabetic macular oedema | Qilu Pharmaceutical Co Ltd | 2023-12-13 | Diabetic macular oedema; Macular Degeneration | Details | |
| Aflibercept | BAY-865321; BAT-86-5321 | Approved | Bayer AG | Eylea, 艾力雅 | United States | Macular Degeneration; Macular Edema | Regeneron Pharmaceuticals Inc | 2011-11-18 | Retinitis Pigmentosa; Choroidal Neovascularization; Carcinoma, Non-Small-Cell Lung; Corneal Neovascularization; Retinopathy of Prematurity; Neoplasm Metastasis; Diabetic Retinopathy; Retinal Vein Occlusion; Macular Degeneration; Vitreous Hemorrhage; Lymphoma, Non-Hodgkin; Retinal Degeneration; Eye Diseases; Diabetes Complications; Colorectal Neoplasms; Prostatic Neoplasms; Diabetes Mellitus, Type 1; Wet Macular Degeneration; Diabetic macular oedema; Multiple Myeloma; Myopia, Degenerative; Retinal Diseases; Colonic Neoplasms; Central Serous Chorioretinopathy; Neoplasms; Diabetes Mellitus, Type 2; Rectal Neoplasms; Choroid Diseases; Glaucoma, Neovascular; Macular Edema; Cataract | Details |
| Ziv-aflibercept | BAY-865321; AVE-0005 | Approved | Sanofi | Zaltrap | United States | Colorectal Neoplasms | Sanofi-Aventis U.S. Llc | 2012-08-03 | Lymphoma; Breast Neoplasms; Urethral Neoplasms; Brain Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Carcinoma, Mucoepidermoid; Gliosarcoma; Astrocytoma; Leukemia, Myeloid, Chronic, Atypical, BCR-ABL Negative; Peritoneal Neoplasms; Ureteral Neoplasms; Lung Neoplasms; Prostatic Neoplasms; Uterine Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms; Carcinoma, Neuroendocrine; Glioma; Lymphoma, Non-Hodgkin; Thyroid Neoplasms; Carcinoma, Squamous Cell; Retinal Vein Occlusion; Neoplasm Metastasis; Melanoma; Carcinoma, Non-Small-Cell Lung; Glioblastoma; Solid tumours; Leiomyosarcoma; Multiple Endocrine Neoplasia Type 1; Leukemia; Carcinoma; Carcinoma, Renal Cell; Carcinoid Tumor; Rectal Neoplasms; Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Leukemia, Myelomonocytic, Chronic; Ovarian Neoplasms; Myelodysplastic Syndromes; Myeloproliferative Disorders; Carcinoma, Transitional Cell; Carcinoma, Papillary; Small Cell Lung Carcinoma; Colonic Neoplasms; Ascites; Lung Diseases; Adenoc | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Aflibercept biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Macular Degeneration | Details | |
| Aflibercept biosimilar (Amgen) | ABP-938 | Phase 3 Clinical | Amgen Inc | Vascular Diseases; Macular Degeneration | Details |
| Aflibercept Biosimilar(Alvotech Swiss) | AVT-06 | Phase 3 Clinical | Alvotech Swiss Ag | Vascular Diseases; Macular Degeneration | Details |
| Aflibercept biosimilar (HEXAL/Sandoz) | SOK-583A1; SOK583A1; SOK-583 | Phase 3 Clinical | Sandoz, Hexal | Macular Degeneration | Details |
| CT-P42 | CT-P42 | Phase 3 Clinical | Celltrion Inc | Macular Edema; Diabetic macular oedema | Details |
| Aflibercept biosimilar (Mabwell) | 9-MW-0813; 9MW-0813; 9MW0813; 9-MW0813 | Phase 3 Clinical | Mabwell (Shanghai) Bioscience Co Ltd | Diabetic macular oedema | Details |
| FYB-203 | FYB-203 | Phase 3 Clinical | Macular Degeneration | Details | |
| Aflibercept biosimilar (Sam Chun Dang Pharm) | SCD-411 | Phase 3 Clinical | Sam Chun Dang Pharm Co Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| Aflibercept biosimilar (Samsung Bioepis) | SB-15; AM003; SB15 | Phase 3 Clinical | Samsung Bioepis Co Ltd | Solid tumours; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details |
| Aflibercept Biosimilar (Boan Biopharma/Luye Pharma) | LY-09004; BA-9101; OT-702 | Phase 3 Clinical | Luye Pharma Group Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| Anti-placental growth factor monoclonal antibody | αPlGF; R-7334; TB-403; RG-7334; THR-317; RO-5323441 | Phase 2 Clinical | Thrombogenics, Bioinvent International Ab | Retinal Telangiectasis; Ovarian Neoplasms; Solid tumours; Medulloblastoma; Macular Edema; Glioblastoma; Colorectal Neoplasms; Diabetic Retinopathy; Diabetes Mellitus; Macular Degeneration; Carcinoma, Hepatocellular | Details |
| 4D-150 | 4D-150 | Phase 2 Clinical | 4d Molecular Therapeutics Inc | Diabetic macular oedema; Macular Degeneration; Diabetic Retinopathy | Details |
| Ziv-aflibercept biosimiliar (Boan Biopharma) | LY-01012; BA-1103 | Phase 1 Clinical | Colorectal Neoplasms | Details | |
| PB-101 | PB-101 | Phase 1 Clinical | Panolos Bioscience Inc | Solid tumours; Stomach Neoplasms; Neoplasms; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| Aflibercept biosimilar (Zein Bioteccnology) | Phase 1 Clinical | Zein Bioteccnology Co Ltd | Diabetic macular oedema | Details | |
| Aflibercept biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Macular Degeneration | Details | |
| Aflibercept biosimilar (Amgen) | ABP-938 | Phase 3 Clinical | Amgen Inc | Vascular Diseases; Macular Degeneration | Details |
| Aflibercept Biosimilar(Alvotech Swiss) | AVT-06 | Phase 3 Clinical | Alvotech Swiss Ag | Vascular Diseases; Macular Degeneration | Details |
| Aflibercept biosimilar (HEXAL/Sandoz) | SOK-583A1; SOK583A1; SOK-583 | Phase 3 Clinical | Sandoz, Hexal | Macular Degeneration | Details |
| CT-P42 | CT-P42 | Phase 3 Clinical | Celltrion Inc | Macular Edema; Diabetic macular oedema | Details |
| Aflibercept biosimilar (Mabwell) | 9-MW-0813; 9MW-0813; 9MW0813; 9-MW0813 | Phase 3 Clinical | Mabwell (Shanghai) Bioscience Co Ltd | Diabetic macular oedema | Details |
| FYB-203 | FYB-203 | Phase 3 Clinical | Macular Degeneration | Details | |
| Aflibercept biosimilar (Sam Chun Dang Pharm) | SCD-411 | Phase 3 Clinical | Sam Chun Dang Pharm Co Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| Aflibercept biosimilar (Samsung Bioepis) | SB-15; AM003; SB15 | Phase 3 Clinical | Samsung Bioepis Co Ltd | Solid tumours; Wet Macular Degeneration; Diabetic macular oedema; Macular Degeneration | Details |
| Aflibercept Biosimilar (Boan Biopharma/Luye Pharma) | LY-09004; BA-9101; OT-702 | Phase 3 Clinical | Luye Pharma Group Ltd | Wet Macular Degeneration; Macular Degeneration | Details |
| Anti-placental growth factor monoclonal antibody | αPlGF; R-7334; TB-403; RG-7334; THR-317; RO-5323441 | Phase 2 Clinical | Thrombogenics, Bioinvent International Ab | Retinal Telangiectasis; Ovarian Neoplasms; Solid tumours; Medulloblastoma; Macular Edema; Glioblastoma; Colorectal Neoplasms; Diabetic Retinopathy; Diabetes Mellitus; Macular Degeneration; Carcinoma, Hepatocellular | Details |
| 4D-150 | 4D-150 | Phase 2 Clinical | 4d Molecular Therapeutics Inc | Diabetic macular oedema; Macular Degeneration; Diabetic Retinopathy | Details |
| Ziv-aflibercept biosimiliar (Boan Biopharma) | LY-01012; BA-1103 | Phase 1 Clinical | Colorectal Neoplasms | Details | |
| PB-101 | PB-101 | Phase 1 Clinical | Panolos Bioscience Inc | Solid tumours; Stomach Neoplasms; Neoplasms; Colorectal Neoplasms; Carcinoma, Hepatocellular | Details |
| Aflibercept biosimilar (Zein Bioteccnology) | Phase 1 Clinical | Zein Bioteccnology Co Ltd | Diabetic macular oedema | Details |
This web search service is supported by Google Inc.