 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| PLU-H5228 | Human | Human PLAU / uPA Protein, His Tag (activated by trypsin) (active enzyme) |  |
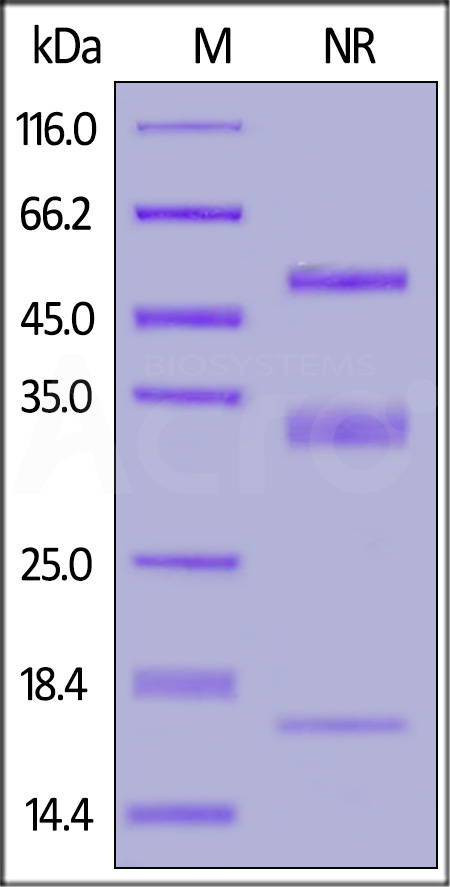
|
|
| PLU-H5229 | Human | Human PLAU / uPA Protein, His Tag |  |
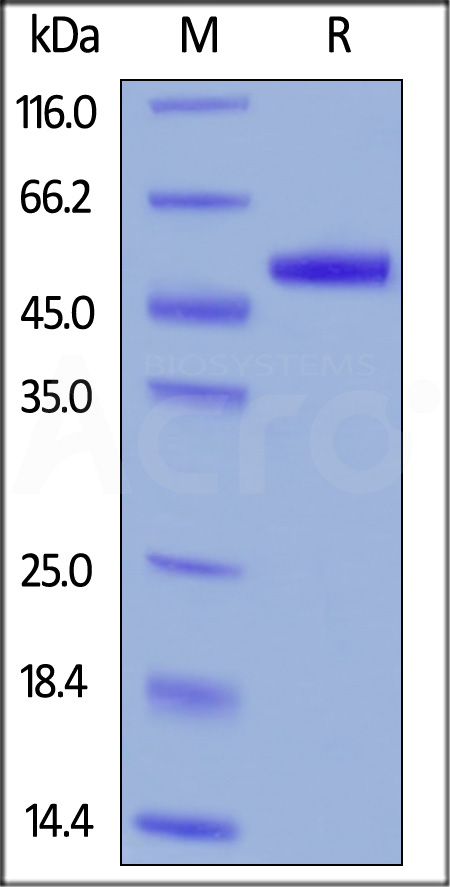
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase biosimilar (Sedico) | Approved | Sedico | Angikinase | Egypt | Pulmonary Embolism; Myocardial Infarction; Thromboembolism | Sedico | 2014-01-01 | Pulmonary Embolism; Myocardial Infarction; Thromboembolism | Details | |
| Tisokinase | AK-124 | Approved | Kowa Co Ltd, Asahi Kasei Corp | Plasvata, Hapase | Japan | Myocardial Infarction; Thrombosis | null | 1991-01-01 | Myocardial Infarction; Thrombosis | Details |
| Urokinase biosimilar (Bharat Serums & Vaccines) | U-FRAG | Approved | Bharat Serums And Vaccines Ltd | Pulmonary Embolism | Details | |||||
| Nasaruplase | GE-0943 | Approved | Mitsubishi Pharma Corp | Tomieze, Thrombolyse | Japan | Myocardial Infarction; Venous Thrombosis | Mitsubishi Pharma Corp | 1992-01-01 | Myocardial Infarction; Venous Thrombosis | Details |
| Urokinase biosimilar (Sedico) | Approved | Sedico | Angikinase | Egypt | Pulmonary Embolism; Myocardial Infarction; Thromboembolism | Sedico | 2014-01-01 | Pulmonary Embolism; Myocardial Infarction; Thromboembolism | Details | |
| Tisokinase | AK-124 | Approved | Kowa Co Ltd, Asahi Kasei Corp | Plasvata, Hapase | Japan | Myocardial Infarction; Thrombosis | null | 1991-01-01 | Myocardial Infarction; Thrombosis | Details |
| Urokinase biosimilar (Bharat Serums & Vaccines) | U-FRAG | Approved | Bharat Serums And Vaccines Ltd | Pulmonary Embolism | Details | |||||
| Nasaruplase | GE-0943 | Approved | Mitsubishi Pharma Corp | Tomieze, Thrombolyse | Japan | Myocardial Infarction; Venous Thrombosis | Mitsubishi Pharma Corp | 1992-01-01 | Myocardial Infarction; Venous Thrombosis | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Urokinase biosimilar (Suzhou Landing Biological Pharmaceutical) | Phase 3 Clinical | Suzhou RxD Biopharmaceutical Co Ltd | ST Elevation Myocardial Infarction | Details | |
| tPA/HisproUK (Thrombolytic Science) | TS-01 | Phase 2 Clinical | Thrombolytic Science | Stroke | Details |
| Upamostat Hydrogen Sulphate (Wilex) | WX-671; LH-011; RHB-107 | Phase 2 Clinical | Pancreatic Neoplasms; Coronavirus Disease 2019 (COVID-19); Breast Neoplasms | Details | |
| Recombinant human annexin 5 protein (Yabao Pharmaceuticals) | SY-005 | Phase 2 Clinical | Yabao Pharmaceutical Group Co Ltd, Lawson Health Research Institute | Sepsis; Coronavirus Infections | Details |
| Recombinant annexin A5 (Annexin Pharmaceuticals) | ANXV; ANXV (Annexin A5); ANXV (Human Recombinant Annexin A5) | Phase 2 Clinical | Annexin Pharmaceuticals AB | Neoplasms; Cardiovascular Diseases; Anemia, Sickle Cell; Retinal Vein Occlusion | Details |
| MSB-03 | MSB-03 | Phase 1 Clinical | Rosacea | Details | |
| Urokinase biosimilar (Suzhou Landing Biological Pharmaceutical) | Phase 3 Clinical | Suzhou RxD Biopharmaceutical Co Ltd | ST Elevation Myocardial Infarction | Details | |
| tPA/HisproUK (Thrombolytic Science) | TS-01 | Phase 2 Clinical | Thrombolytic Science | Stroke | Details |
| Upamostat Hydrogen Sulphate (Wilex) | WX-671; LH-011; RHB-107 | Phase 2 Clinical | Pancreatic Neoplasms; Coronavirus Disease 2019 (COVID-19); Breast Neoplasms | Details | |
| Recombinant human annexin 5 protein (Yabao Pharmaceuticals) | SY-005 | Phase 2 Clinical | Yabao Pharmaceutical Group Co Ltd, Lawson Health Research Institute | Sepsis; Coronavirus Infections | Details |
| Recombinant annexin A5 (Annexin Pharmaceuticals) | ANXV; ANXV (Annexin A5); ANXV (Human Recombinant Annexin A5) | Phase 2 Clinical | Annexin Pharmaceuticals AB | Neoplasms; Cardiovascular Diseases; Anemia, Sickle Cell; Retinal Vein Occlusion | Details |
| MSB-03 | MSB-03 | Phase 1 Clinical | Rosacea | Details |
This web search service is supported by Google Inc.