 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| MIF-H82E7 | Human | Biotinylated Human MIF Protein, His,Avitag™ |  |
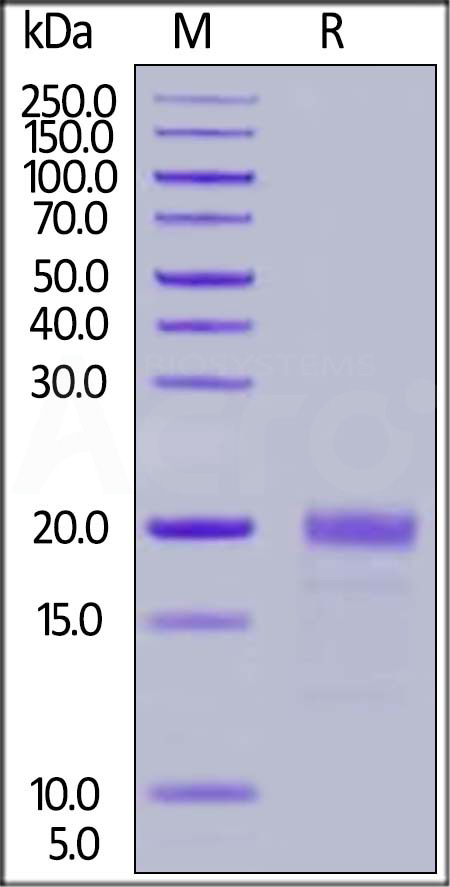
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Ibudilast | AV-411; KC-404; MN-166 | Approved | Ketas, Pinatos, Eyevinal | Japan | Asthma; Multiple Sclerosis; Neuralgia | Kyorin Pharmaceutical Co Ltd | HIV Infections; Substance-Related Disorders; Alcohol-Induced Disorders; Headache Disorders, Secondary; Diabetic Neuropathies; Opioid-Related Disorders; Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Pneumonia, Viral; Alcoholism; Asthma; Neuralgia; Amyotrophic Lateral Sclerosis | Details | ||
| Ibudilast | AV-411; KC-404; MN-166 | Approved | Ketas, Pinatos, Eyevinal | Japan | Asthma; Multiple Sclerosis; Neuralgia | Kyorin Pharmaceutical Co Ltd | HIV Infections; Substance-Related Disorders; Alcohol-Induced Disorders; Headache Disorders, Secondary; Diabetic Neuropathies; Opioid-Related Disorders; Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Pneumonia, Viral; Alcoholism; Asthma; Neuralgia; Amyotrophic Lateral Sclerosis | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| IPG-1094 | IPG-1094; IPG-001 | Phase 2 Clinical | Nanjing Immunophage Biotech Co Ltd | Solid tumours; Hematologic Neoplasms; Multiple Sclerosis; Multiple Myeloma; Lupus Nephritis; Psoriasis; Brain Neoplasms; Brain metastases | Details |
| IPG-1094 | IPG-1094; IPG-001 | Phase 2 Clinical | Nanjing Immunophage Biotech Co Ltd | Solid tumours; Hematologic Neoplasms; Multiple Sclerosis; Multiple Myeloma; Lupus Nephritis; Psoriasis; Brain Neoplasms; Brain metastases | Details |
This web search service is supported by Google Inc.