 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
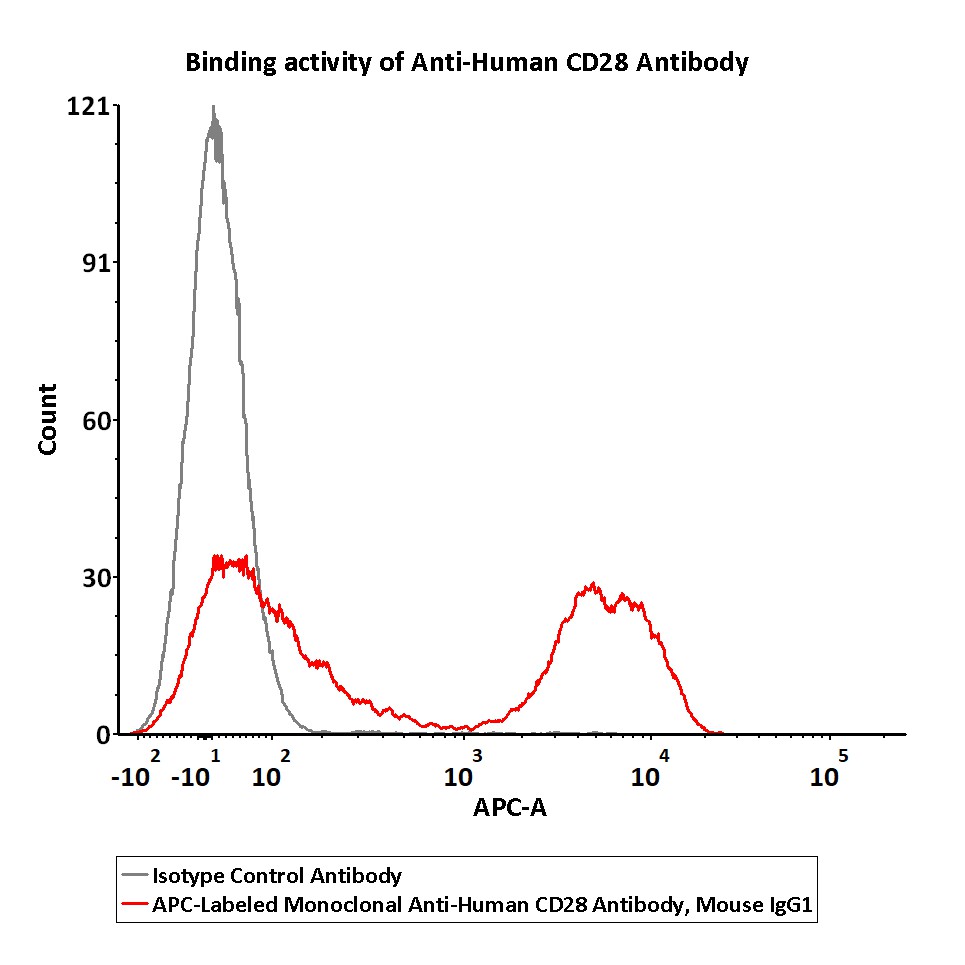
Flow cytometric analysis of Human peripheral blood lymphocytes respectively staining with APC-Labeled Monoclonal Anti-Human CD28 Antibody, Mouse IgG1 (Cat. No. CD8-AHFC1) at 1:50 dilution (2 μL of the antibody stock solution corresponds to labeling of 1e6 cells in a final volume of 100 µL), compared with isotype control antibody. APC signal was used to evaluate the binding activity (QC tested).
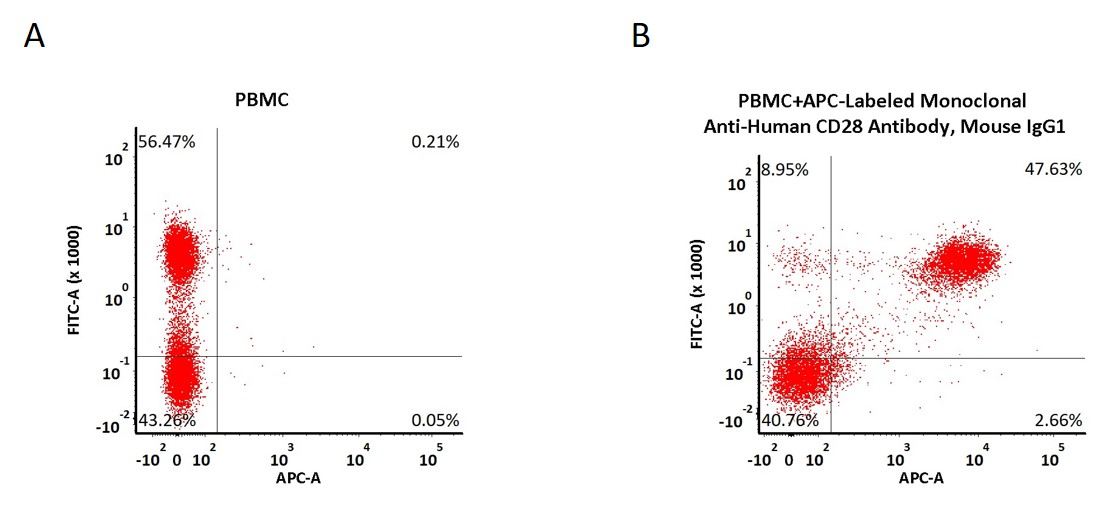
Non-specificity of APC-Labeled Monoclonal Anti-Human CD28 Antibody, Mouse IgG1 (Cat. No. CD8-AHFC1) binding to CD3- cells present in human PBMC. Human PBMCs were simultaneously stained with FITC anti-human CD3 Antibody and APC-Labeled Monoclonal Anti-Human CD28 Antibody, Mouse IgG1 (2 μL of the antibody stock solution corresponds to labeling of 5e5 cells in a final volume of 100 µL), washed and then analyzed with FACS. FITC negative signals and APC positive signals were used to evaluate the non-specific binding activity to human CD3- cells (Routinely tested).
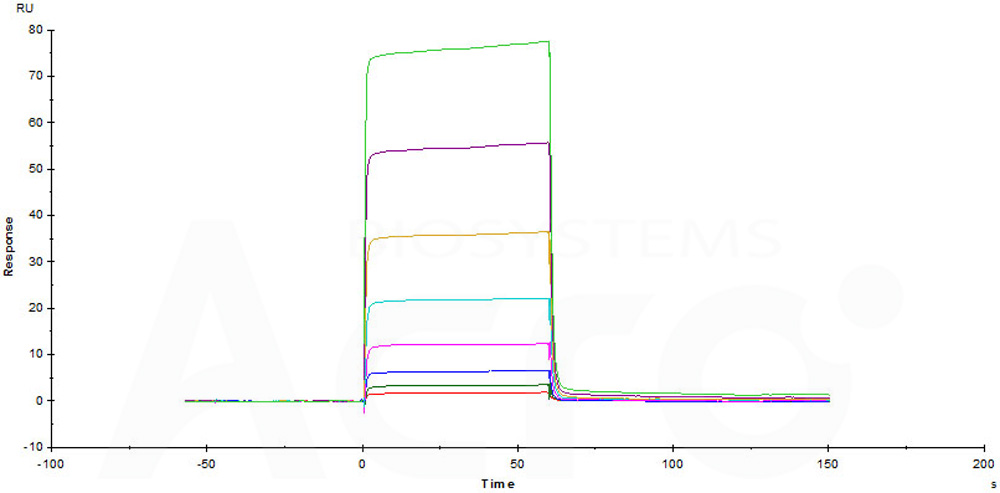
Immobilized Human / Cynomolgus / Rhesus macaque CD28, Fc Tag (Cat. No. CD8-H525a) on CM5 Chip can bind Human B7-1, His Tag (Cat. No. B71-H5228) with an affinity constant of 5.29 μM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Reltecimod | P2-TA; AB-103 | Phase 3 Clinical | Atox Bio Ltd | Peritonitis; Acute Kidney Injury; Fasciitis, Necrotizing; Fournier Gangrene; Soft Tissue Infections | Details |
| Nezastomig | REGN-5678 | Phase 2 Clinical | Carcinoma, Renal Cell; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| Abatacept (Orban Biotech) | Phase 2 Clinical | Orban Biotech, The National Institute Of Diabetes And Digestive And Kidney Diseases | Diabetes Mellitus, Type 1 | Details | |
| Lulizumab pegol | Lulizumab; BMS-931699 | Phase 2 Clinical | Bristol-Myers Squibb Company | Lupus Vulgaris; Rejection of renal transplantation; Sjogren-Larsson Syndrome | Details |
| Acazicolcept | ALPN-101 | Phase 2 Clinical | Alpine Immune Sciences Inc | Graft vs Host Disease; Autoimmune Diseases; Lupus Erythematosus, Systemic; Lymphoproliferative Disorders; Inflammation | Details |
| FR-104 | FR-104; JNJ-3133; VEL-101 | Phase 2 Clinical | Effimune | Rejection of renal transplantation; Arthritis, Rheumatoid; Postoperative Complications | Details |
| TC-510 | TC-510 | Phase 2 Clinical | Tcr2 Therapeutics Inc | Ovarian Neoplasms; Solid tumours; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Mesothelioma; Cholangiocarcinoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| REGN-7075 | REGN-7075 | Phase 2 Clinical | Solid tumours | Details | |
| REGN-5668 | REGN-5668 | Phase 2 Clinical | Ovarian Neoplasms; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms | Details | |
| ATLCAR.k.CD28 cells (UNC Lineberger Comprehensive Cancer Center) | Phase 1 Clinical | Unc Lineberger Comprehensive Cancer Center | Lymphoma, B-Cell, Marginal Zone; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details | |
| SAR-442257 | SAR-442257 | Phase 1 Clinical | Sanofi | Neoplasms | Details |
| Autologous CD19CAR-CD28-CD3zeta-EGFRt-expressing Tcm-enriched T cells (City of Hope Medical Center) | Phase 1 Clinical | City Of Hope National Medical Center | Lymphoma, Non-Hodgkin | Details | |
| JNJ-1493 | JNJ-1493 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Lymphoma, B-Cell | Details |
| JNJ-9401 | JNJ-9401 | Phase 1 Clinical | Xencor Inc | Prostatic Neoplasms | Details |
| JNJ-87189401 | JNJ-87189401 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms | Details |
| REGN5837 | REGN5837; REGN-5837 | Phase 1 Clinical | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| CC-312 | CC-312 | Phase 1 Clinical | CytoCares (Shanghai) Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| RG-6333 | RO-7443904 | Phase 1 Clinical | F. Hoffmann-La Roche Ag | Lymphoma, Non-Hodgkin | Details |
| XmAb-808 | XmAb808 | Phase 1 Clinical | Xencor Inc | Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Prostatic Neoplasms, Castration-Resistant; Colorectal Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| SAR-443216 | SAR-443216 | Phase 1 Clinical | Sanofi | Solid tumours; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Lung Neoplasms | Details |
| CD19 PD-1/CD28 CAR-T Cell Therapy (Second Affiliated Hospital School Of Zhejiang University School Of Medicine) | Phase 1 Clinical | Second Affiliated Hospital Of Zhejiang University School Of Medicine | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Primary mediastinal B cell lymphoma | Details | |
| FPT-155 | CD80-Fc; FPT-155 | Phase 1 Clinical | Five Prime Therapeutics Inc | Solid tumours | Details |
| Theralizumab | TAB-08; TABO-8 | Phase 1 Clinical | Theramab Llc | Solid tumours; Carcinoma, Merkel Cell; Arthritis, Rheumatoid; Psoriasis; Lupus Erythematosus, Systemic | Details |
| B7-2/GM-CSF cancer gene therapy | CIT | Phase 1 Clinical | Radient | Neoplasms | Details |
| InHeAb-01 | InHeAb-01; bsAB | Clinical | University Hospital Tuebingen | Neoplasms | Details |
| Reltecimod | P2-TA; AB-103 | Phase 3 Clinical | Atox Bio Ltd | Peritonitis; Acute Kidney Injury; Fasciitis, Necrotizing; Fournier Gangrene; Soft Tissue Infections | Details |
| Nezastomig | REGN-5678 | Phase 2 Clinical | Carcinoma, Renal Cell; Prostatic Neoplasms, Castration-Resistant; Prostatic Neoplasms | Details | |
| Abatacept (Orban Biotech) | Phase 2 Clinical | Orban Biotech, The National Institute Of Diabetes And Digestive And Kidney Diseases | Diabetes Mellitus, Type 1 | Details | |
| Lulizumab pegol | Lulizumab; BMS-931699 | Phase 2 Clinical | Bristol-Myers Squibb Company | Lupus Vulgaris; Rejection of renal transplantation; Sjogren-Larsson Syndrome | Details |
| Acazicolcept | ALPN-101 | Phase 2 Clinical | Alpine Immune Sciences Inc | Graft vs Host Disease; Autoimmune Diseases; Lupus Erythematosus, Systemic; Lymphoproliferative Disorders; Inflammation | Details |
| FR-104 | FR-104; JNJ-3133; VEL-101 | Phase 2 Clinical | Effimune | Rejection of renal transplantation; Arthritis, Rheumatoid; Postoperative Complications | Details |
| TC-510 | TC-510 | Phase 2 Clinical | Tcr2 Therapeutics Inc | Ovarian Neoplasms; Solid tumours; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Mesothelioma; Cholangiocarcinoma; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| REGN-7075 | REGN-7075 | Phase 2 Clinical | Solid tumours | Details | |
| REGN-5668 | REGN-5668 | Phase 2 Clinical | Ovarian Neoplasms; Peritoneal Neoplasms; Endometrial Neoplasms; Fallopian Tube Neoplasms | Details | |
| ATLCAR.k.CD28 cells (UNC Lineberger Comprehensive Cancer Center) | Phase 1 Clinical | Unc Lineberger Comprehensive Cancer Center | Lymphoma, B-Cell, Marginal Zone; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details | |
| SAR-442257 | SAR-442257 | Phase 1 Clinical | Sanofi | Neoplasms | Details |
| Autologous CD19CAR-CD28-CD3zeta-EGFRt-expressing Tcm-enriched T cells (City of Hope Medical Center) | Phase 1 Clinical | City Of Hope National Medical Center | Lymphoma, Non-Hodgkin | Details | |
| JNJ-1493 | JNJ-1493 | Phase 1 Clinical | Xencor Inc | Hematologic Neoplasms; Lymphoma, B-Cell | Details |
| JNJ-9401 | JNJ-9401 | Phase 1 Clinical | Xencor Inc | Prostatic Neoplasms | Details |
| JNJ-87189401 | JNJ-87189401 | Phase 1 Clinical | Janssen Research & Development Llc | Prostatic Neoplasms | Details |
| REGN5837 | REGN5837; REGN-5837 | Phase 1 Clinical | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| CC-312 | CC-312 | Phase 1 Clinical | CytoCares (Shanghai) Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| RG-6333 | RO-7443904 | Phase 1 Clinical | F. Hoffmann-La Roche Ag | Lymphoma, Non-Hodgkin | Details |
| XmAb-808 | XmAb808 | Phase 1 Clinical | Xencor Inc | Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Prostatic Neoplasms, Castration-Resistant; Colorectal Neoplasms; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| SAR-443216 | SAR-443216 | Phase 1 Clinical | Sanofi | Solid tumours; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Lung Neoplasms | Details |
| CD19 PD-1/CD28 CAR-T Cell Therapy (Second Affiliated Hospital School Of Zhejiang University School Of Medicine) | Phase 1 Clinical | Second Affiliated Hospital Of Zhejiang University School Of Medicine | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Primary mediastinal B cell lymphoma | Details | |
| FPT-155 | CD80-Fc; FPT-155 | Phase 1 Clinical | Five Prime Therapeutics Inc | Solid tumours | Details |
| Theralizumab | TAB-08; TABO-8 | Phase 1 Clinical | Theramab Llc | Solid tumours; Carcinoma, Merkel Cell; Arthritis, Rheumatoid; Psoriasis; Lupus Erythematosus, Systemic | Details |
| B7-2/GM-CSF cancer gene therapy | CIT | Phase 1 Clinical | Radient | Neoplasms | Details |
| InHeAb-01 | InHeAb-01; bsAB | Clinical | University Hospital Tuebingen | Neoplasms | Details |
This web search service is supported by Google Inc.