 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
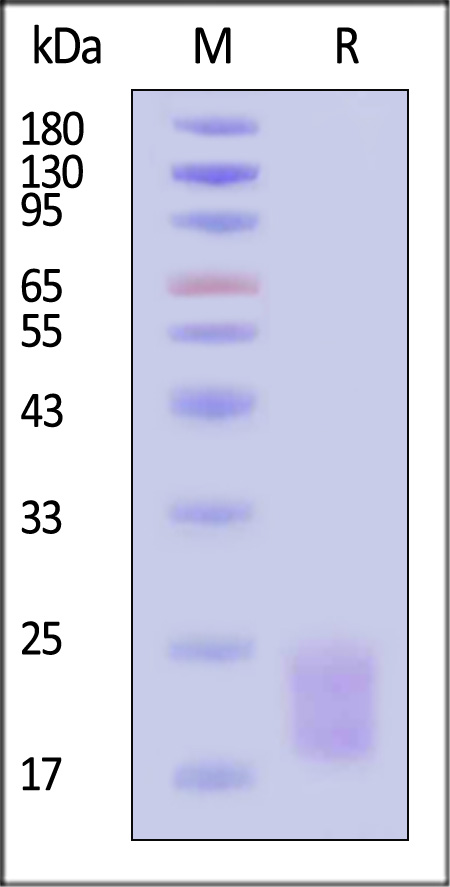
| ILF-H52W6 | Human | Human IL-17A&IL-17F Heterodimer Protein, Twin Strep&His Tag (MALS verified) |  |
|
|
| ILF-H82W1 | Human | Biotinylated Human IL-17A&IL-17F Protein, Avitag™,His Tag |  |

|

Human IL17RA & IL17RC Protein, Fc Tag&Fc Tag (Cat. No. ILC-H5257) captured on CM5 chip via anti-human IgG Fc antibody can bind Human IL-17A&IL-17F Heterodimer Protein, Twin Strep&His Tag (Cat. No. ILF-H52W6) with an affinity constant of 50.8 pM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Ixekizumab | LY-2439821 | Approved | Eli Lilly And Company | 拓咨, Taltz | United States | Psoriasis | Eli Lilly And Company | 2016-03-22 | Diabetes Mellitus, Type 1; Non-radiographic axial spondyloarthritis; Depressive Disorder, Major; Pyoderma Gangrenosum; Spondylarthritis; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Psoriasis; Arthritis, Psoriatic; Lichen Planus; Pemphigoid, Bullous; Pityriasis Rubra Pilaris; Plaque psoriasis | Details |
| Virulizin | Approved | Aptose | Virulizin, Virulizin-2 gamma | Mexico | Sarcoma, Kaposi; Melanoma; Carcinoma, Renal Cell; Colorectal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Uterine Cervical Neoplasms | null | 1997-01-01 | Carcinoma, Renal Cell; Stomach Neoplasms; Pancreatic Neoplasms; Colorectal Neoplasms; Sarcoma, Kaposi; Melanoma; Uterine Cervical Neoplasms | Details | |
| Secukinumab | NVP-AIN-457; AIN-457; KB-03303A | Approved | Novartis Pharma Ag | 可善挺, Scapho, Cosentyx | Japan | Arthritis, Psoriatic; Psoriasis | Novartis Pharma Ag | 2014-12-26 | Hidradenitis Suppurativa; Polymyalgia Rheumatica; Ichthyosis, Lamellar; Psoriasis; Tendinopathy; Hyperkeratosis, Epidermolytic; Arthritis, Psoriatic; Tendon Injuries; Asthma; Lichen Planus; Arthritis, Juvenile; Uveitis; Arthritis; Inflammation; Plaque psoriasis; Behcet Syndrome; Crohn Disease; Gastroenteritis; Dermatitis, Atopic; Ichthyosiform Erythroderma, Congenital; Spondylitis, Ankylosing; Non-radiographic axial spondyloarthritis; Necrobiosis Lipoidica; Diabetes Mellitus, Type 1; Giant Cell Arteritis; Alopecia Areata; Pyoderma Gangrenosum; Lupus Erythematosus, Discoid; Metabolic Dysfunction-Associated Steatotic Liver Disease; Inflammatory Bowel Diseases; Multiple Sclerosis, Relapsing-Remitting; Rotator Cuff Injuries; Spondylarthritis; Arthritis, Rheumatoid; Spondylarthropathies; Netherton Syndrome; Ichthyosis; Lupus Nephritis; Graves Ophthalmopathy; Multiple Sclerosis | Details |
| Ixekizumab | LY-2439821 | Approved | Eli Lilly And Company | 拓咨, Taltz | United States | Psoriasis | Eli Lilly And Company | 2016-03-22 | Diabetes Mellitus, Type 1; Non-radiographic axial spondyloarthritis; Depressive Disorder, Major; Pyoderma Gangrenosum; Spondylarthritis; Arthritis, Rheumatoid; Spondylitis, Ankylosing; Psoriasis; Arthritis, Psoriatic; Lichen Planus; Pemphigoid, Bullous; Pityriasis Rubra Pilaris; Plaque psoriasis | Details |
| Virulizin | Approved | Aptose | Virulizin, Virulizin-2 gamma | Mexico | Sarcoma, Kaposi; Melanoma; Carcinoma, Renal Cell; Colorectal Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Uterine Cervical Neoplasms | null | 1997-01-01 | Carcinoma, Renal Cell; Stomach Neoplasms; Pancreatic Neoplasms; Colorectal Neoplasms; Sarcoma, Kaposi; Melanoma; Uterine Cervical Neoplasms | Details | |
| Secukinumab | NVP-AIN-457; AIN-457; KB-03303A | Approved | Novartis Pharma Ag | 可善挺, Scapho, Cosentyx | Japan | Arthritis, Psoriatic; Psoriasis | Novartis Pharma Ag | 2014-12-26 | Hidradenitis Suppurativa; Polymyalgia Rheumatica; Ichthyosis, Lamellar; Psoriasis; Tendinopathy; Hyperkeratosis, Epidermolytic; Arthritis, Psoriatic; Tendon Injuries; Asthma; Lichen Planus; Arthritis, Juvenile; Uveitis; Arthritis; Inflammation; Plaque psoriasis; Behcet Syndrome; Crohn Disease; Gastroenteritis; Dermatitis, Atopic; Ichthyosiform Erythroderma, Congenital; Spondylitis, Ankylosing; Non-radiographic axial spondyloarthritis; Necrobiosis Lipoidica; Diabetes Mellitus, Type 1; Giant Cell Arteritis; Alopecia Areata; Pyoderma Gangrenosum; Lupus Erythematosus, Discoid; Metabolic Dysfunction-Associated Steatotic Liver Disease; Inflammatory Bowel Diseases; Multiple Sclerosis, Relapsing-Remitting; Rotator Cuff Injuries; Spondylarthritis; Arthritis, Rheumatoid; Spondylarthropathies; Netherton Syndrome; Ichthyosis; Lupus Nephritis; Graves Ophthalmopathy; Multiple Sclerosis | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| SSGJ-608 | 608; SSGJ-608 | Phase 3 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Non-radiographic axial spondyloarthritis; Spondylitis, Ankylosing; Spondylarthritis; Psoriasis; Plaque psoriasis | Details |
| QX002N | QX-002N | Phase 3 Clinical | Qyuns Therapeutics Co Ltd | Spondylitis, Ankylosing; Asthma | Details |
| Roconkibart | JS005 | Phase 3 Clinical | Shanghai Junshi Biosciences Co Ltd | Non-radiographic axial spondyloarthritis; Spondylarthritis; Spondylitis, Ankylosing; Psoriasis; Plaque psoriasis | Details |
| Gumokimab | AK-111 | Phase 3 Clinical | Zhongshan Akeso Biopharma Co Ltd | Non-radiographic axial spondyloarthritis; Spondylarthritis; Spondylitis, Ankylosing; Skin Diseases; Psoriasis; Plaque psoriasis | Details |
| Izokibep | IMG-020; IMG020; ABY-035; AFB-035 | Phase 3 Clinical | Affibody Ab | Non-radiographic axial spondyloarthritis; Spondylitis, Ankylosing; Psoriasis; Arthritis, Psoriatic; Hidradenitis Suppurativa; Uveitis; Plaque psoriasis | Details |
| Recombinant human IL-17A/F humanized antibody (Livzon Pharmaceutical Group) | LZM-012; LZM012; XKH004; XKH-004 | Phase 3 Clinical | Beijing Kanova Biomedical Technology Co Ltd, Livzon Pharmaceutical Group Inc | Spondylitis, Ankylosing; Psoriasis; Plaque psoriasis | Details |
| Secukinumab biosimilar (Bio-Thera Solutions) | BAT-2306 | Phase 3 Clinical | Bio-Thera Solutions Ltd | Autoimmune Diseases; Psoriasis; Plaque psoriasis | Details |
| DMT-310 | DMT-310 | Phase 3 Clinical | Dermata | Acne Vulgaris; Rosacea | Details |
| CJM-112 | CJM-112 | Phase 2 Clinical | Novartis Pharma Ag | Acne Vulgaris; Triple Negative Breast Neoplasms; Multiple Sclerosis; Multiple Myeloma; Psoriasis; Asthma; Colorectal Neoplasms; Hidradenitis Suppurativa; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| Sonelokimab | M-1095; ALX-0761; MSB-0010841 | Phase 2 Clinical | Ablynx Nv | Psoriasis; Arthritis, Psoriatic; Hidradenitis Suppurativa | Details |
| SIM-0335 | SIM-0335; SIM-335 | Phase 2 Clinical | BCY Pharm Co Ltd | Plaque psoriasis | Details |
| SCT-650C | SCT650C; SCT-650-C; SCT-650C | Phase 2 Clinical | SinoCelltech Ltd | Non-radiographic axial spondyloarthritis; Autoimmune Diseases; Plaque psoriasis | Details |
| Isomyosamine | MYMD-1 | Phase 2 Clinical | Mymd Pharmaceuticals Inc | Depression; Anxiety; Hashimoto Disease; Frailty; Coronavirus Disease 2019 (COVID-19); Arthritis, Rheumatoid; Healthy Aging; Inflammation; Sarcopenia | Details |
| CNTO-6785 | CNTO-6785; FTC-001 | Phase 1 Clinical | Morphosys Ag, Johnson & Johnson Innovative Medicine | Arthritis, Rheumatoid; Spondylitis, Ankylosing; Psoriasis; Arthritis, Psoriatic; Pulmonary Disease, Chronic Obstructive | Details |
| JNJ-61178104 | JNJ-61178104 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Autoimmune Diseases | Details |
| Secukinumab biosimilar (BioRay Pharmaceutical) | BR-201 | Phase 1 Clinical | BioRay Pharmaceutical Co Ltd | Autoimmune Diseases; Spondylitis, Ankylosing; Psoriasis; Plaque psoriasis | Details |
| JNJ-63823539 | JNJ-63823539 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Arthritis, Rheumatoid | Details |
| Secukinumab Biosimilar(Celltrion) | CT-P55 | Phase 1 Clinical | Celltrion Inc | Plaque psoriasis | Details |
| Secukinumab biosimilar (CSPC Pharmaceutical Group) | SYS6012; SYS-6012 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd | Plaque psoriasis | Details |
| Secukinumab biosimilar (Gan & Lee) | GLR1023; GLR-1023 | Phase 1 Clinical | Gan & Lee Pharmaceuticals | Plaque psoriasis | Details |
| anti-IL-17A humanized monoclonal antibody(Fontacea ) | Phase 1 Clinical | Shandong Fangtansi Biopharmaceutical Co Ltd | Plaque psoriasis | Details | |
| CMAB-015 | CMAB015; CMAB-015 | Phase 1 Clinical | Taizhou Mabtech Pharmaceutical Co Ltd | Spondylitis, Ankylosing; Plaque psoriasis | Details |
| DC-853 | DC-853 | Phase 1 Clinical | Dice Therapeutics Inc | Immune System Diseases | Details |
| LEO-153339 | LEO-153339 | Phase 1 Clinical | Leo Pharma Inc | Details | |
| HY-1770 | HY1770; HT-17; HT17; HY-1770 | Phase 1 Clinical | Suzhou Pharmavan Co Ltd | Dermatitis, Atopic; Plaque psoriasis | Details |
| ZL-1102 | ZL-1102; CB-001 | Phase 1 Clinical | Crescendo Biologics Ltd, Zai Lab (Shanghai) Co Ltd | Psoriasis | Details |
| UCB-0159 | UCB-0159 | Phase 1 Clinical | Ucb Sa | Autoimmune Diseases | Details |
| SSGJ-608 | 608; SSGJ-608 | Phase 3 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Non-radiographic axial spondyloarthritis; Spondylitis, Ankylosing; Spondylarthritis; Psoriasis; Plaque psoriasis | Details |
| QX002N | QX-002N | Phase 3 Clinical | Qyuns Therapeutics Co Ltd | Spondylitis, Ankylosing; Asthma | Details |
| Roconkibart | JS005 | Phase 3 Clinical | Shanghai Junshi Biosciences Co Ltd | Non-radiographic axial spondyloarthritis; Spondylarthritis; Spondylitis, Ankylosing; Psoriasis; Plaque psoriasis | Details |
| Gumokimab | AK-111 | Phase 3 Clinical | Zhongshan Akeso Biopharma Co Ltd | Non-radiographic axial spondyloarthritis; Spondylarthritis; Spondylitis, Ankylosing; Skin Diseases; Psoriasis; Plaque psoriasis | Details |
| Izokibep | IMG-020; IMG020; ABY-035; AFB-035 | Phase 3 Clinical | Affibody Ab | Non-radiographic axial spondyloarthritis; Spondylitis, Ankylosing; Psoriasis; Arthritis, Psoriatic; Hidradenitis Suppurativa; Uveitis; Plaque psoriasis | Details |
| Recombinant human IL-17A/F humanized antibody (Livzon Pharmaceutical Group) | LZM-012; LZM012; XKH004; XKH-004 | Phase 3 Clinical | Beijing Kanova Biomedical Technology Co Ltd, Livzon Pharmaceutical Group Inc | Spondylitis, Ankylosing; Psoriasis; Plaque psoriasis | Details |
| Secukinumab biosimilar (Bio-Thera Solutions) | BAT-2306 | Phase 3 Clinical | Bio-Thera Solutions Ltd | Autoimmune Diseases; Psoriasis; Plaque psoriasis | Details |
| DMT-310 | DMT-310 | Phase 3 Clinical | Dermata | Acne Vulgaris; Rosacea | Details |
| CJM-112 | CJM-112 | Phase 2 Clinical | Novartis Pharma Ag | Acne Vulgaris; Triple Negative Breast Neoplasms; Multiple Sclerosis; Multiple Myeloma; Psoriasis; Asthma; Colorectal Neoplasms; Hidradenitis Suppurativa; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| Sonelokimab | M-1095; ALX-0761; MSB-0010841 | Phase 2 Clinical | Ablynx Nv | Psoriasis; Arthritis, Psoriatic; Hidradenitis Suppurativa | Details |
| SIM-0335 | SIM-0335; SIM-335 | Phase 2 Clinical | BCY Pharm Co Ltd | Plaque psoriasis | Details |
| SCT-650C | SCT650C; SCT-650-C; SCT-650C | Phase 2 Clinical | SinoCelltech Ltd | Non-radiographic axial spondyloarthritis; Autoimmune Diseases; Plaque psoriasis | Details |
| Isomyosamine | MYMD-1 | Phase 2 Clinical | Mymd Pharmaceuticals Inc | Depression; Anxiety; Hashimoto Disease; Frailty; Coronavirus Disease 2019 (COVID-19); Arthritis, Rheumatoid; Healthy Aging; Inflammation; Sarcopenia | Details |
| CNTO-6785 | CNTO-6785; FTC-001 | Phase 1 Clinical | Morphosys Ag, Johnson & Johnson Innovative Medicine | Arthritis, Rheumatoid; Spondylitis, Ankylosing; Psoriasis; Arthritis, Psoriatic; Pulmonary Disease, Chronic Obstructive | Details |
| JNJ-61178104 | JNJ-61178104 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Autoimmune Diseases | Details |
| Secukinumab biosimilar (BioRay Pharmaceutical) | BR-201 | Phase 1 Clinical | BioRay Pharmaceutical Co Ltd | Autoimmune Diseases; Spondylitis, Ankylosing; Psoriasis; Plaque psoriasis | Details |
| JNJ-63823539 | JNJ-63823539 | Phase 1 Clinical | Johnson & Johnson Innovative Medicine | Arthritis, Rheumatoid | Details |
| Secukinumab Biosimilar(Celltrion) | CT-P55 | Phase 1 Clinical | Celltrion Inc | Plaque psoriasis | Details |
| Secukinumab biosimilar (CSPC Pharmaceutical Group) | SYS6012; SYS-6012 | Phase 1 Clinical | CSPC Pharmaceutical Group Ltd | Plaque psoriasis | Details |
| Secukinumab biosimilar (Gan & Lee) | GLR1023; GLR-1023 | Phase 1 Clinical | Gan & Lee Pharmaceuticals | Plaque psoriasis | Details |
| anti-IL-17A humanized monoclonal antibody(Fontacea ) | Phase 1 Clinical | Shandong Fangtansi Biopharmaceutical Co Ltd | Plaque psoriasis | Details | |
| CMAB-015 | CMAB015; CMAB-015 | Phase 1 Clinical | Taizhou Mabtech Pharmaceutical Co Ltd | Spondylitis, Ankylosing; Plaque psoriasis | Details |
| DC-853 | DC-853 | Phase 1 Clinical | Dice Therapeutics Inc | Immune System Diseases | Details |
| LEO-153339 | LEO-153339 | Phase 1 Clinical | Leo Pharma Inc | Details | |
| HY-1770 | HY1770; HT-17; HT17; HY-1770 | Phase 1 Clinical | Suzhou Pharmavan Co Ltd | Dermatitis, Atopic; Plaque psoriasis | Details |
| ZL-1102 | ZL-1102; CB-001 | Phase 1 Clinical | Crescendo Biologics Ltd, Zai Lab (Shanghai) Co Ltd | Psoriasis | Details |
| UCB-0159 | UCB-0159 | Phase 1 Clinical | Ucb Sa | Autoimmune Diseases | Details |
This web search service is supported by Google Inc.