 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
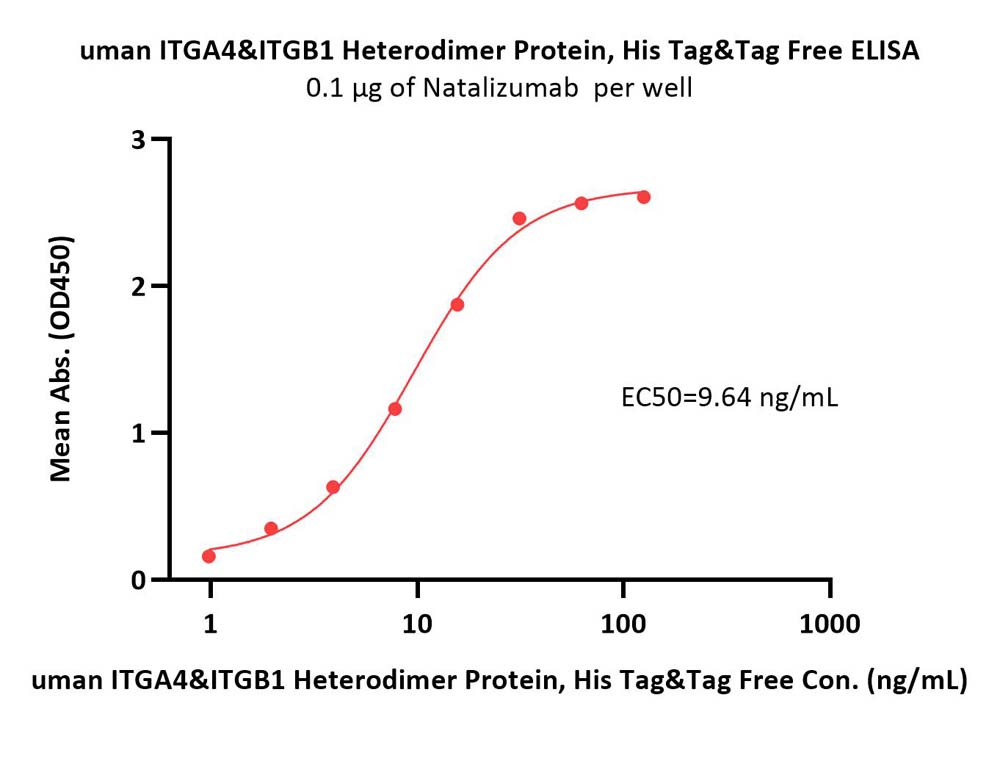
Immobilized Natalizumab at 1 μg/mL (100 μL/well) can bind Human ITGA4&ITGB1 Heterodimer Protein, His Tag&Tag Free (Cat. No. IT1-H52W1) with a linear range of 1-16 ng/mL (QC tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Carotegrast methyl | AJM-300 | Approved | Ea Pharma, Eisai Co Ltd | Carogra | Japan | Colitis, Ulcerative | Ea Pharma Co Ltd | 2022-03-28 | Colitis, Ulcerative | Details |
| Natalizumab biosimilar(Polpharma Biologics) | PB-006; DST-356A1 | Approved | Polpharma Biologics Sa | Tyruko | United States | Crohn Disease; Multiple Sclerosis | Sandoz Inc | 2023-08-24 | Multiple Sclerosis, Relapsing-Remitting; Multiple Sclerosis; Crohn Disease | Details |
| Natalizumab | BG-0002; TY-21.6; AN-10022; BG-00002; AN-100226; BG-0002-E | Approved | Biogen Inc, Perrigo Llc | Tysabri, Antegran, Antegren | United States | Multiple Sclerosis; Crohn Disease | Biogen Idec | 2004-11-23 | Multiple Sclerosis, Relapsing-Remitting; Epilepsies, Partial; Arthritis, Rheumatoid; Graft vs Host Disease; Stroke; Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Multiple Myeloma; Demyelinating Diseases; Myositis, Inclusion Body; Crohn Disease | Details |
| Carotegrast methyl | AJM-300 | Approved | Ea Pharma, Eisai Co Ltd | Carogra | Japan | Colitis, Ulcerative | Ea Pharma Co Ltd | 2022-03-28 | Colitis, Ulcerative | Details |
| Natalizumab biosimilar(Polpharma Biologics) | PB-006; DST-356A1 | Approved | Polpharma Biologics Sa | Tyruko | United States | Crohn Disease; Multiple Sclerosis | Sandoz Inc | 2023-08-24 | Multiple Sclerosis, Relapsing-Remitting; Multiple Sclerosis; Crohn Disease | Details |
| Natalizumab | BG-0002; TY-21.6; AN-10022; BG-00002; AN-100226; BG-0002-E | Approved | Biogen Inc, Perrigo Llc | Tysabri, Antegran, Antegren | United States | Multiple Sclerosis; Crohn Disease | Biogen Idec | 2004-11-23 | Multiple Sclerosis, Relapsing-Remitting; Epilepsies, Partial; Arthritis, Rheumatoid; Graft vs Host Disease; Stroke; Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Multiple Myeloma; Demyelinating Diseases; Myositis, Inclusion Body; Crohn Disease | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| AS-101 | PRX-0001/AS101; WAX-120337; IVAX-Q-101; IVX-Q-101; CB-06-02; AS-101; PRX-001; PRX-0002/AS101 | Phase 2 Clinical | Biomas Ltd | Alopecia; HIV Infections; Warts; Myelodysplastic Syndromes; Psoriasis; Leukemia, Myeloid, Acute; Thrombocytopenia; Macular Degeneration; Dermatitis, Atopic; Condylomata Acuminata | Details |
| ATL-1102 | ATL-1102; ATL/TV-1102; ISIS-107248; TV-1102 | Phase 2 Clinical | Ionis Pharmaceuticals Inc | Multiple Sclerosis; Muscular Dystrophy, Duchenne | Details |
| Alintegimod | 7HP349; 7-HP-349; 7HP-349 | Phase 2 Clinical | 7 Hills Pharma LLC | Kidney Neoplasms; Liver Neoplasms; Solid tumours; Mismatch Repair Deficient Cancer; Carcinoma, Renal Cell; Neoplasms; Skin Neoplasms; Mesothelioma; Colorectal Neoplasms; Melanoma; Neoplasm Metastasis; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| LLP2A alendronate | Phase 1 Clinical | University Of California | Osteonecrosis; Osteoporosis; Bone Diseases, Metabolic; Bone Diseases | Details | |
| RBx-7796 | RBx-7796 | Clinical | Sun Pharmaceutical Industries Ltd | Rhinitis, Allergic; Asthma | Details |
| AS-101 | PRX-0001/AS101; WAX-120337; IVAX-Q-101; IVX-Q-101; CB-06-02; AS-101; PRX-001; PRX-0002/AS101 | Phase 2 Clinical | Biomas Ltd | Alopecia; HIV Infections; Warts; Myelodysplastic Syndromes; Psoriasis; Leukemia, Myeloid, Acute; Thrombocytopenia; Macular Degeneration; Dermatitis, Atopic; Condylomata Acuminata | Details |
| ATL-1102 | ATL-1102; ATL/TV-1102; ISIS-107248; TV-1102 | Phase 2 Clinical | Ionis Pharmaceuticals Inc | Multiple Sclerosis; Muscular Dystrophy, Duchenne | Details |
| Alintegimod | 7HP349; 7-HP-349; 7HP-349 | Phase 2 Clinical | 7 Hills Pharma LLC | Kidney Neoplasms; Liver Neoplasms; Solid tumours; Mismatch Repair Deficient Cancer; Carcinoma, Renal Cell; Neoplasms; Skin Neoplasms; Mesothelioma; Colorectal Neoplasms; Melanoma; Neoplasm Metastasis; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| LLP2A alendronate | Phase 1 Clinical | University Of California | Osteonecrosis; Osteoporosis; Bone Diseases, Metabolic; Bone Diseases | Details | |
| RBx-7796 | RBx-7796 | Clinical | Sun Pharmaceutical Industries Ltd | Rhinitis, Allergic; Asthma | Details |
This web search service is supported by Google Inc.