 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
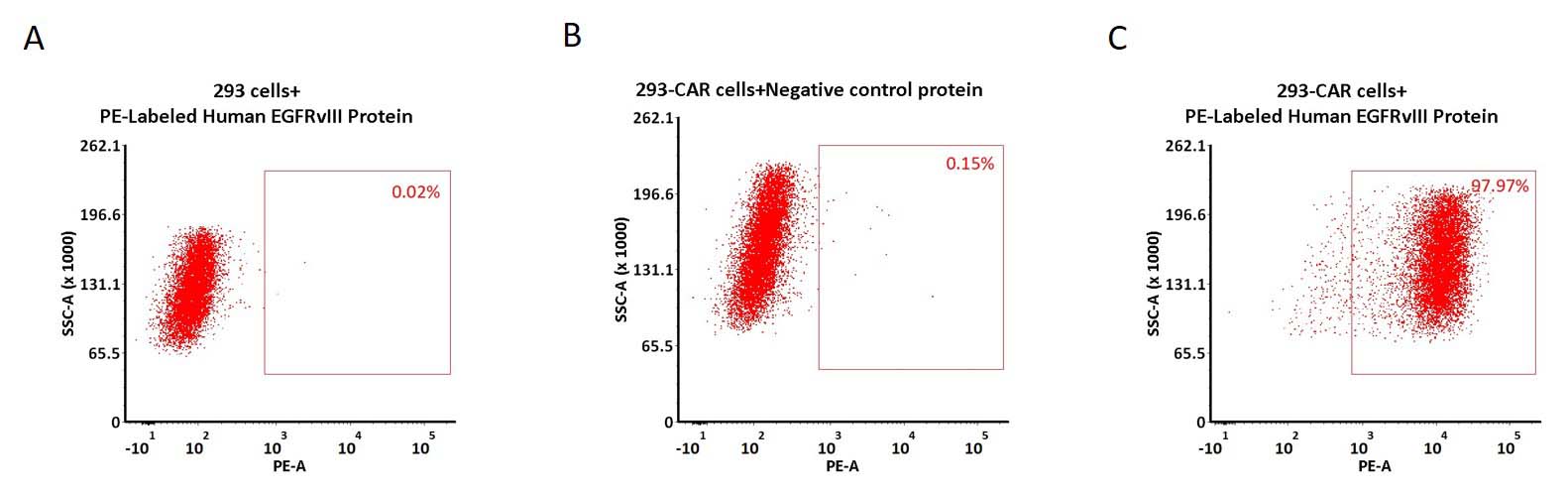
5e5 of anti-EGFRvIII CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human EGFRvIII, His Tag (Cat. No. EGI-HP2H6) and negative control protein respectively (Fig. C and B), and non-transfected 293 cells were used as a control (Fig. A). PE signal was used to evaluate the binding activity (QC tested).

Immobilized Human EGFRvIII, His Tag (Cat. No. EGI-H52H4) at 1 μg/mL (100 μL/well) can bind Anti-EGFRvIII Antibody , Human IgG1 with a linear range of 0.1-2 ng/mL (QC tested).

The purity of Human EGFRvIII, Fc Tag (Cat. No. EGI-H5255) is more than 90% and the molecular weight of this protein is around 150-184 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Anti-EGFRvIII-CAR | EGFRvIII CAR; Anti-EGFRvIII-CAR | Phase 2 Clinical | National Cancer Institute | Glioblastoma; Brain Neoplasms; Gliosarcoma; Glioma | Details |
| Recombinant anti-EGFR chimeric monoclonal antibody (Shanghai CP Guojian) | CPGJ-602; 602; CPGJ602; CPGJ 602 | Phase 2 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Colorectal Neoplasms | Details |
| WSD-0922 | WSD-0922; WSD0922 | Phase 1 Clinical | Wayshine Biopharm International Ltd | Glioblastoma; Neoplasms; Astrocytoma; Carcinoma, Non-Small-Cell Lung | Details |
| EGFRvIII-directed CAR-T cell tharapy (Novartis/University of Pennsylvania) | LXF-821 | Phase 1 Clinical | University Of Pennsylvania, Novartis Pharma Ag | Glioblastoma | Details |
| EGFRvIII-CAR | Phase 1 Clinical | Duke University Medical Center | Glioblastoma | Details | |
| Autologous Anti-EGFRvIII synNotch Receptor Induced Anti-EphA2/IL-13R alpha2 CAR (E-SYNC) T Cells therapy(California Institute For Regenerative Medicine) | Phase 1 Clinical | California Institute For Regenerative Medicine, National Cancer Institute | Glioblastoma | Details | |
| Targeted EGFRvIII autochimeric antigen receptor T cell therapy (DCTY) | DCTY0801; DCTY-0801 | Phase 1 Clinical | Beijing DCTY Biotech Co Ltd | Glioblastoma | Details |
| SNC-109 | SNC109; SNC-109 | Phase 1 Clinical | Shanghai Simnova Biotechnology Co Ltd | Glioblastoma | Details |
| RO-7428731 | RO-7428731; RG-6156 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Glioblastoma | Details |
| EGFRvIII Targeted Chimeric Antigen Receptor CAR-T Cell Therapy (Chembrain) | Phase 1 Clinical | Chembrain Ltd | Glioblastoma; Glioma | Details | |
| hEGFRvIII-CD3 Bi-scFv | Phase 1 Clinical | Duke University | Glioblastoma; Glioma | Details | |
| GNC-039 | GNC-039 | Phase 1 Clinical | Solid tumours; Hematologic Neoplasms; Glioma; Neoplasm Metastasis | Details | |
| Etevritamab | AMG-596 | Phase 1 Clinical | Amgen Inc | Glioblastoma | Details |
| Anti-EGFRvIII-CAR | EGFRvIII CAR; Anti-EGFRvIII-CAR | Phase 2 Clinical | National Cancer Institute | Glioblastoma; Brain Neoplasms; Gliosarcoma; Glioma | Details |
| Recombinant anti-EGFR chimeric monoclonal antibody (Shanghai CP Guojian) | CPGJ-602; 602; CPGJ602; CPGJ 602 | Phase 2 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Colorectal Neoplasms | Details |
| WSD-0922 | WSD-0922; WSD0922 | Phase 1 Clinical | Wayshine Biopharm International Ltd | Glioblastoma; Neoplasms; Astrocytoma; Carcinoma, Non-Small-Cell Lung | Details |
| EGFRvIII-directed CAR-T cell tharapy (Novartis/University of Pennsylvania) | LXF-821 | Phase 1 Clinical | University Of Pennsylvania, Novartis Pharma Ag | Glioblastoma | Details |
| EGFRvIII-CAR | Phase 1 Clinical | Duke University Medical Center | Glioblastoma | Details | |
| Autologous Anti-EGFRvIII synNotch Receptor Induced Anti-EphA2/IL-13R alpha2 CAR (E-SYNC) T Cells therapy(California Institute For Regenerative Medicine) | Phase 1 Clinical | California Institute For Regenerative Medicine, National Cancer Institute | Glioblastoma | Details | |
| Targeted EGFRvIII autochimeric antigen receptor T cell therapy (DCTY) | DCTY0801; DCTY-0801 | Phase 1 Clinical | Beijing DCTY Biotech Co Ltd | Glioblastoma | Details |
| SNC-109 | SNC109; SNC-109 | Phase 1 Clinical | Shanghai Simnova Biotechnology Co Ltd | Glioblastoma | Details |
| RO-7428731 | RO-7428731; RG-6156 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Glioblastoma | Details |
| EGFRvIII Targeted Chimeric Antigen Receptor CAR-T Cell Therapy (Chembrain) | Phase 1 Clinical | Chembrain Ltd | Glioblastoma; Glioma | Details | |
| hEGFRvIII-CD3 Bi-scFv | Phase 1 Clinical | Duke University | Glioblastoma; Glioma | Details | |
| GNC-039 | GNC-039 | Phase 1 Clinical | Solid tumours; Hematologic Neoplasms; Glioma; Neoplasm Metastasis | Details | |
| Etevritamab | AMG-596 | Phase 1 Clinical | Amgen Inc | Glioblastoma | Details |
| Anti-EGFRvIII-CAR | EGFRvIII CAR; Anti-EGFRvIII-CAR | Phase 2 Clinical | National Cancer Institute | Glioblastoma; Brain Neoplasms; Gliosarcoma; Glioma | Details |
| Recombinant anti-EGFR chimeric monoclonal antibody (Shanghai CP Guojian) | CPGJ-602; 602; CPGJ602; CPGJ 602 | Phase 2 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Colorectal Neoplasms | Details |
| WSD-0922 | WSD-0922; WSD0922 | Phase 1 Clinical | Wayshine Biopharm International Ltd | Glioblastoma; Neoplasms; Astrocytoma; Carcinoma, Non-Small-Cell Lung | Details |
| EGFRvIII-directed CAR-T cell tharapy (Novartis/University of Pennsylvania) | LXF-821 | Phase 1 Clinical | University Of Pennsylvania, Novartis Pharma Ag | Glioblastoma | Details |
| EGFRvIII-CAR | Phase 1 Clinical | Duke University Medical Center | Glioblastoma | Details | |
| Autologous Anti-EGFRvIII synNotch Receptor Induced Anti-EphA2/IL-13R alpha2 CAR (E-SYNC) T Cells therapy(California Institute For Regenerative Medicine) | Phase 1 Clinical | California Institute For Regenerative Medicine, National Cancer Institute | Glioblastoma | Details | |
| Targeted EGFRvIII autochimeric antigen receptor T cell therapy (DCTY) | DCTY0801; DCTY-0801 | Phase 1 Clinical | Beijing DCTY Biotech Co Ltd | Glioblastoma | Details |
| SNC-109 | SNC109; SNC-109 | Phase 1 Clinical | Shanghai Simnova Biotechnology Co Ltd | Glioblastoma | Details |
| RO-7428731 | RO-7428731; RG-6156 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Glioblastoma | Details |
| EGFRvIII Targeted Chimeric Antigen Receptor CAR-T Cell Therapy (Chembrain) | Phase 1 Clinical | Chembrain Ltd | Glioblastoma; Glioma | Details | |
| hEGFRvIII-CD3 Bi-scFv | Phase 1 Clinical | Duke University | Glioblastoma; Glioma | Details | |
| GNC-039 | GNC-039 | Phase 1 Clinical | Solid tumours; Hematologic Neoplasms; Glioma; Neoplasm Metastasis | Details | |
| Etevritamab | AMG-596 | Phase 1 Clinical | Amgen Inc | Glioblastoma | Details |
| Anti-EGFRvIII-CAR | EGFRvIII CAR; Anti-EGFRvIII-CAR | Phase 2 Clinical | National Cancer Institute | Glioblastoma; Brain Neoplasms; Gliosarcoma; Glioma | Details |
| Recombinant anti-EGFR chimeric monoclonal antibody (Shanghai CP Guojian) | CPGJ-602; 602; CPGJ602; CPGJ 602 | Phase 2 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Colorectal Neoplasms | Details |
| WSD-0922 | WSD-0922; WSD0922 | Phase 1 Clinical | Wayshine Biopharm International Ltd | Glioblastoma; Neoplasms; Astrocytoma; Carcinoma, Non-Small-Cell Lung | Details |
| EGFRvIII-directed CAR-T cell tharapy (Novartis/University of Pennsylvania) | LXF-821 | Phase 1 Clinical | University Of Pennsylvania, Novartis Pharma Ag | Glioblastoma | Details |
| EGFRvIII-CAR | Phase 1 Clinical | Duke University Medical Center | Glioblastoma | Details | |
| Autologous Anti-EGFRvIII synNotch Receptor Induced Anti-EphA2/IL-13R alpha2 CAR (E-SYNC) T Cells therapy(California Institute For Regenerative Medicine) | Phase 1 Clinical | California Institute For Regenerative Medicine, National Cancer Institute | Glioblastoma | Details | |
| Targeted EGFRvIII autochimeric antigen receptor T cell therapy (DCTY) | DCTY0801; DCTY-0801 | Phase 1 Clinical | Beijing DCTY Biotech Co Ltd | Glioblastoma | Details |
| SNC-109 | SNC109; SNC-109 | Phase 1 Clinical | Shanghai Simnova Biotechnology Co Ltd | Glioblastoma | Details |
| RO-7428731 | RO-7428731; RG-6156 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Glioblastoma | Details |
| EGFRvIII Targeted Chimeric Antigen Receptor CAR-T Cell Therapy (Chembrain) | Phase 1 Clinical | Chembrain Ltd | Glioblastoma; Glioma | Details | |
| hEGFRvIII-CD3 Bi-scFv | Phase 1 Clinical | Duke University | Glioblastoma; Glioma | Details | |
| GNC-039 | GNC-039 | Phase 1 Clinical | Solid tumours; Hematologic Neoplasms; Glioma; Neoplasm Metastasis | Details | |
| Etevritamab | AMG-596 | Phase 1 Clinical | Amgen Inc | Glioblastoma | Details |
This web search service is supported by Google Inc.