 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Insights > Development and Metastasis of Cancer: Regulation and Remodeling of the Tumor Microenvironment The progression and metastasis of cancer are intimately connected to the regulation and remodeling of the tumor microenvironment (TME). The extracellular matrix (ECM), a complex network of macromolecules, supports cells by governing key processes like anchorage, migration, and proliferation. Degradation of the ECM is associated with diseases like cancer, where tumors exploit ECM remodeling to create a microenvironment that promotes tumorigenesis and metastasis. This remodeling not only provides structural support but also affects cellular pathways, signal transduction, intercellular communication, and cell proliferation and death regulation.1 ECM remodeling encompasses four primary processes: (1) ECM deposition, which alters component abundance and affects both biochemical and mechanical properties; (2) chemical modification at the post-translational adjusts biochemical traits and structure; (3) proteolytic degradation releases bioactive fragments and factors, overcoming cellular barriers; (4) force-mediated physical remodeling, aligning fibers and enabling cell migration.1
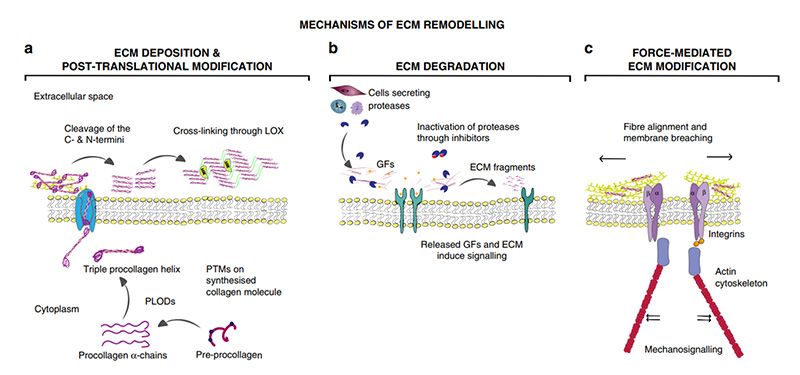
Mechanisms of ECM remodeling.
Matrix metalloproteinases (MMPs), zinc-dependent endopeptidases, primarily degrade ECM components during tissue remodeling, playing a critical role in ECM dynamics. 2 Commonly overexpressed in the tumor microenvironment (TME), MMPs cleave the ECM proteins, which releases Matrix-bound growth factors (GFs) and cytokines, and ECM fragments, including matrilines and also remove barriers for cell migration, and facilitating the migration and dissemination of tumor cells. 1
In humans, 23 structurally related MMPs are known, which can be categorized into six groups based on substrate specificity and structural differences: collagenases, gelatinases, stromelysins, matrilysins, metalloelastase, and membrane-type MMPs.3 The proteolytic degradation of ECM components by MMPs has complex and dual effects on tumorigenesis. For instance, MMP-8 exemplifies this complexity by both promoting and inhibiting tumors. Elevated MMP-8 levels are linked to reduced survival in ovarian and hepatocellular cancers, its low expression correlates with poor prognosis in oral tongue squamous cell carcinoma. These context-dependent outcomes underscore the intricate influence of ECM-degrading enzymes on cancer advancement. 4-6 As such, MMPs play a dual role in the TME.
Moreover, it is essential to recognize that MMPs' roles in tumor progression extend beyond the proteolysis of the ECM. For example, MMP-3 can bind and inhibit noncanonical Wnt5b, thereby enhancing canonical Wnt signaling.7 Similarly, MMP-14 (MT1-MMP) facilitates basal cell extrusion during EMT and promotes basal cell mitosis through non-proteolytic mechanisms. 8
As agents capable of remodeling ECM, MMPs have been linked to a wide variety of biological processes. Growing evidence indicates that elevated MMP expression is intricately linked with tumor proliferation, invasion, and metastasis.3 In diverse cancers, including colorectal, breast, and lung cancers, markedly increased MMP expression has been documented. These elevated MMP levels not only facilitate the migration and dissemination of tumor cells but also promote tumor growth and angiogenesis by releasing growth factors. Moreover, different members of the MMP family have unique roles.4
• MMP-1 is implicated in diseases like lung emphysema, arthritis, and various cancers, promoting cancer cell invasion and migration. Increased MMP-1 expression has been observed in cancers such as oral and bladder, and is linked to a poor prognosis.9 MMP-1 promotes colorectal cancer malignancy via EMT and Akt signaling, and its upregulation along with VEGF-C signifies poor prognosis in esophageal carcinoma. MMP-1 also activates other MMPs, such as MMP-9 and MMP-2, and is crucial in processes like angiogenesis, embryogenesis, morphogenesis, and wound repair.10
• Tumor cells can produce or induce MMP-2 in neighboring cells, fueling tumor progression. MMP-2 degrades the ECM, boosting cancer cell migration and impacting growth signaling, apoptosis resistance, and angiogenesis.11 Overexpression is common in cancers like bladder, breast, and lung. MMP-2 is pivotal in the proteolytic breakdown of ECM components and basement membrane, making it a target for tissue repair and combating cancer metastasis.
• MMP-3 has dual roles in malignancy, acting as both a tumor promoter and inhibitor depending on its substrates. It inhibits angiogenesis by breaking down plasminogen and type VIII collagen, but can also stimulate cancer cell proliferation. Increased MMP-3 expression is associated with several cancers, including breast, oral squamous cell, prostate, and non-small-cell lung cancer, indicating its potential as a prognostic factor and therapeutic target.9
• Overexpression of MMP-7 can promote the invasive, metastatic, and angiogenic characteristics of cancer cells, making it an important target for anticancer drug design. Inhibitors targeting MMP-7 have shown potential therapeutic effects in preclinical studies for multiple types of cancer. 9
• MMP-9 is associated with immune evasion in hepatocellular carcinoma, and inhibiting MMP-9 or using anti-MMP-9 monoclonal antibodies can enhance the effectiveness of immunotherapy. In colorectal cancer and adenomas, concentrations of MMP-7, MMP-9, TIMP-1, and TIMP-2 are significantly increased, playing core roles in the development of colorectal cancer. Upregulation of chemokines CCL27 and CXCL10 can enhance the invasiveness and migration of cancer cells through activation of specific MMPs like MMP-7 and MMP-9.12
• MMP-14, a crucial player in epithelial-mesenchymal transition (EMT), enhances cell migration and invasion by degrading the ECM. Its role in regulating cell-matrix interactions promotes the transition from epithelial to mesenchymal states, boosting migratory capacity. Overexpression of MMP-14 is associated with cancer invasion and metastasis, making it a critical regulator in tumor development. Notably, MMP-14 activates other MMPs, such as MMP-2 and MMP-9, further promoting ECM degradation and tumor cell invasion. Its presence serves as a prognostic indicator for various cancers, correlating with unfavorable outcomes and cancer spread to distant sites in the body.9
Over time, MMPs have been evaluated for their role in cancer progression, migration, and metastasis. Accordingly, various MMPs have become attractive therapeutic targets for anticancer drug development. Scientists have developed a series of MMP inhibitors. These inhibitors block the activity or expression levels of MMPs, inhibiting tumor cell invasion and metastasis. Some MMP inhibitors have entered clinical trials and have shown promising therapeutic effects in certain types of cancer.
Numerous drug delivery systems leveraging MMP responsiveness have been explored. Liu et al. contributed to this field by developing a vesicular drug delivery system, ITC⊂N-G-C, which features a polymeric shell cross-linked by MMP-2-degradable peptides. This design ensures systemic stability and enables tumor-specific drug release through MMP-2-mediated shell degradation, thereby enhancing the therapeutic efficacy in cancer treatment.13
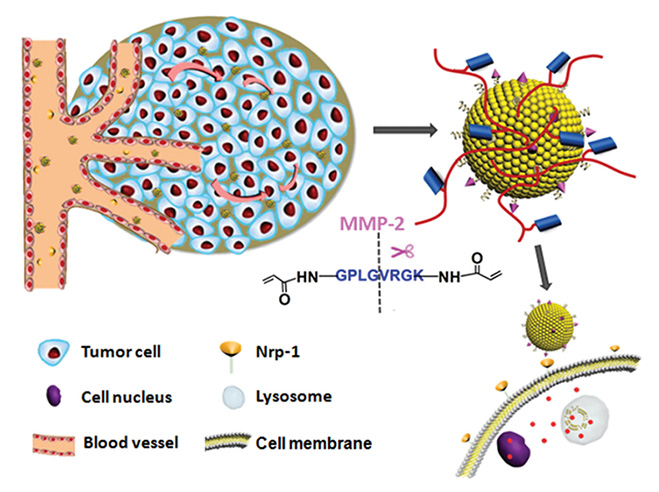
Schematic illustrations of the mechanism of the matrix metalloproteinase (MMPs)-responsive drug‐delivery system (ITC⊂N-G-C) in the tumor microenvironment.
The research team, led by Hu et al., designed a gelatin-based multistage drug delivery system with surface-modified nanoparticles (RGD-DOX-DGL-GNP) around 34.3 nm in size. The gelatin core, acting as an MMP-2 substrate, allowed for size reduction from 155.4 nm to smaller dimensions in the presence of MMP-2. In vitro experiments verified the nanoparticles' size reduction upon MMP-2 exposure, while tumor penetration tests illustrated enhanced deep tumor infiltration capabilities. In vivo assessments highlighted the system's exceptional tumor targeting and potent anti-tumor effects.14
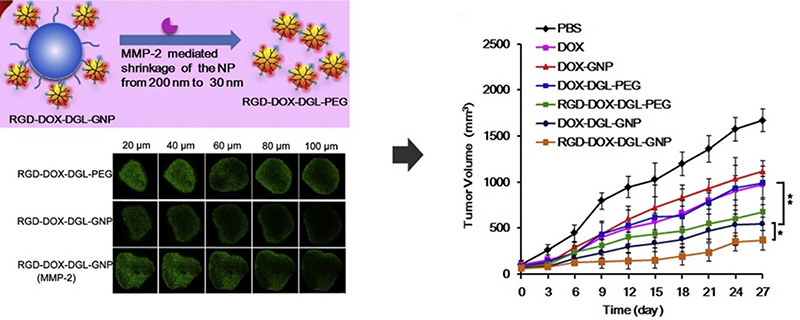
Besides, Novel nanotechnology approaches have been developed in cancer treatment. For instance, a nanofiber system integrating DOX with the KGFRWR peptide, a derivative of amyloid ß protein, demonstrated effectiveness in reducing hepatocellular carcinoma growth. Here, DOX's cytotoxicity targets tumor cells while the KGFRWR peptide suppresses MMP activity.15
Likewise, metallofullerenol nanoparticles like Gd@C82(OH)22 have shown the ability to inhibit MMP-2 and MMP-9 synthesis through allosteric mechanisms.16
Matrix metalloproteinases (MMPs) play a crucial role in reshaping the tumor microenvironment and cancer development. By studying the mechanisms of MMP action and developing effective MMP inhibitors, new pathways for cancer treatment can be explored.
We offer a spectrum of purified MMP proteins (>90% purity verified by SEC-MALS) for diverse applications, such as immunization, antibody screening, immunology, drug hit-lead discovery, and enzyme research.
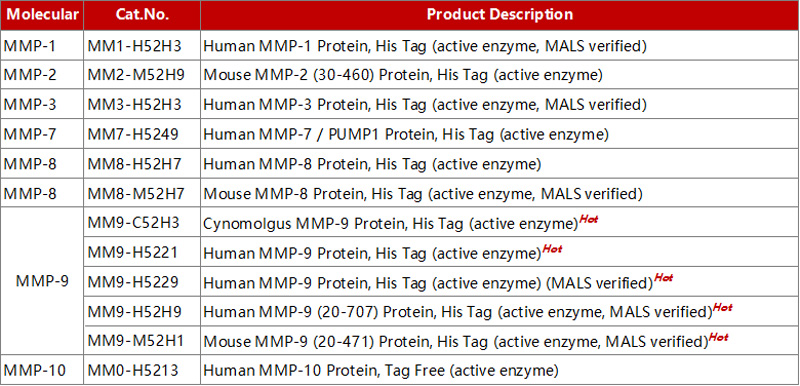
Explore our MMP protein family products
• High purity (>90%) verified by SDS-PAGE
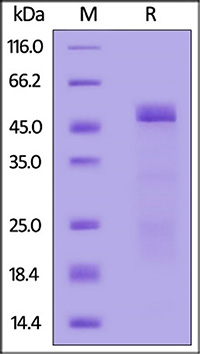
Mouse MMP-2 (30-460) Protein, His Tag (active enzyme) (Cat. No. MM2-M52H9) on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 85%.
• Protein homogeneity and high purity (>90%) verified by SEC-MALS
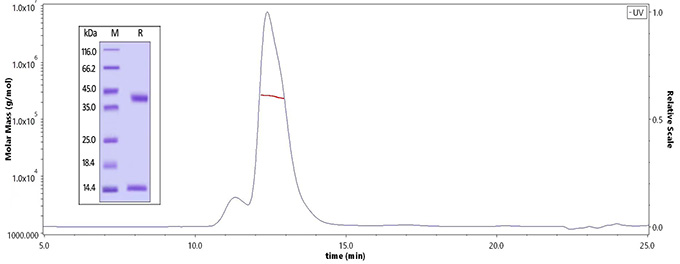
The purity of Human MMP-9, His Tag (Cat. No. MM9-H5229) is more than 90% and the molecular weight of this protein is around 50-70 kDa verified by SEC-MALS. The purity of the protein is greater than 95% verified by SDS-PAGE.
• Enzyme activity data
| Cat. No. | Human MMP-9 Protein, His Tag (active enzyme) (MALS verified) | |
| Substrate | Mca-PLGL-Dpa-AR-NH2 | |
| Enzyme Activity (pmol/min/μg) | ACRO product (Cat. No. MM9-H5229) | Competitor R |
| > 3,000 (QC tested) | > 1,300 | |
| Request a protocol | ||
| Cat. No. | Human MMP-7 / PUMP1 Protein, His Tag (active enzyme) | |
| Substrate | Mca-PLGL-Dpa-AR-NH2 | |
| Enzyme Activity (pmol/min/μg) | ACRO product (Cat. No. MM7-H5249) | Competitor R |
| > 600 (QC tested) | > 600 | |
| Request a protocol | ||
| Cat. No. | Human MMP-3 Protein, His Tag (active enzyme, MALS verified) | |
| Substrate | Mca-RPKPVE-Nval-WRK(Dnp)-NH2 | |
| Enzyme Activity (pmol/min/μg) | ACRO product (Cat. No. MM3-H52H3) | Competitor R |
| > 422 (QC tested) | > 150 | |
| Request a protocol | ||
>>> Click here to explore more tumor microenvironment targets
>>> Click here to explore more enzymes
1. Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K.J. et al. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 11, 5120 (2020).
2. Abdool, A.Y.; Abbas, L.; Sebaei, T.; Schmitt, E.; Sikora, A. How can we design an inhibitor with an enhanced binding affinity that is selective for MMP12? In Protein Modeling Reports 4; NSUWorks: Fort Lauderdale, FL, USA, 2021.
3. Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. S1), 177–183.
4. Stadlmann, S. et al. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur. J. Cancer 39, 2499–2505 (2003).
5. Qin, G. et al. Reciprocal activation between MMP-8 and TGF-beta1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Lett. 374, 85–95 (2016).
6. Astrom, P. et al. The interplay of matrix metalloproteinase-8, transforming growth factor-beta1 and vascular endothelial growth factor-C cooperatively contributes to the aggressiveness of oral tongue squamous cell carcinoma. Br. J. Cancer 117, 1007–1016 (2017).
7. Kessenbrock, K., Plaks, V. & Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
8. Andrieu, C. et al. MMP14 is required for delamination of chick neural crest cells independently of its catalytic activity. Development 147, dev183954 (2020).
9. Almutairi S, Kalloush HM, Manoon NA, Bardaweel SK. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013-2023). Molecules. 2023 Jul 21;28(14):5567.
10. Galt, S.W.; Lindemann, S.; Allen, L.; Medd, D.J.; Falk, J.M.; McIntyre, T.M.; Prescott, S.M.; Kraiss, L.W.; Zimmerman, G.A.; Weyrich, A.S. Outside-in signals delivered by matrix metalloproteinase-1 regulate platelet function. Circ. Res. 2002, 90, 1093–1099.
11. Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol./Hematol. 2019, 137, 57–83.
12. Mustafa S, Koran S, AlOmair L. Insights Into the Role of Matrix Metalloproteinases in Cancer and its Various Therapeutic Aspects: A Review. Front Mol Biosci. 2022 Sep 29;9:896099.
13. Liu Y, Zhang D, Qiao ZY, Qi GB, Liang XJ, Chen XG, Wang H. A Peptide-Network Weaved Nanoplatform with Tumor Microenvironment Responsiveness and Deep Tissue Penetration Capability for Cancer Therapy. Adv Mater. 2015 Sep 9;27(34):5034-42.
14. Hu G, Zhang H, Zhang L, Ruan S, He Q, Gao H. Integrin-mediated active tumor targeting and tumor microenvironment response dendrimer-gelatin nanoparticles for drug delivery and tumor treatment. Int J Pharm. 2015 Dec 30;496(2):1057-68.
15. Ji Y., Xiao Y., Xu L., He J., Qian C., Li W., et al. (2018). Drug-Bearing Supramolecular MMP Inhibitor Nanofibers for Inhibition of Metastasis and Growth of Liver Cancer. Adv. Sci. 5, 1700867. 10.1002/advs.201700867.
16. Kang S.-g., Zhou G., Yang P., Liu Y., Sun B., Huynh T., et al. (2012). Molecular Mechanism of Pancreatic Tumor Metastasis Inhibition by Gd@C 82 (OH) 22 and its Implication for De Novo Design of Nanomedicine.
This web search service is supported by Google Inc.