 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
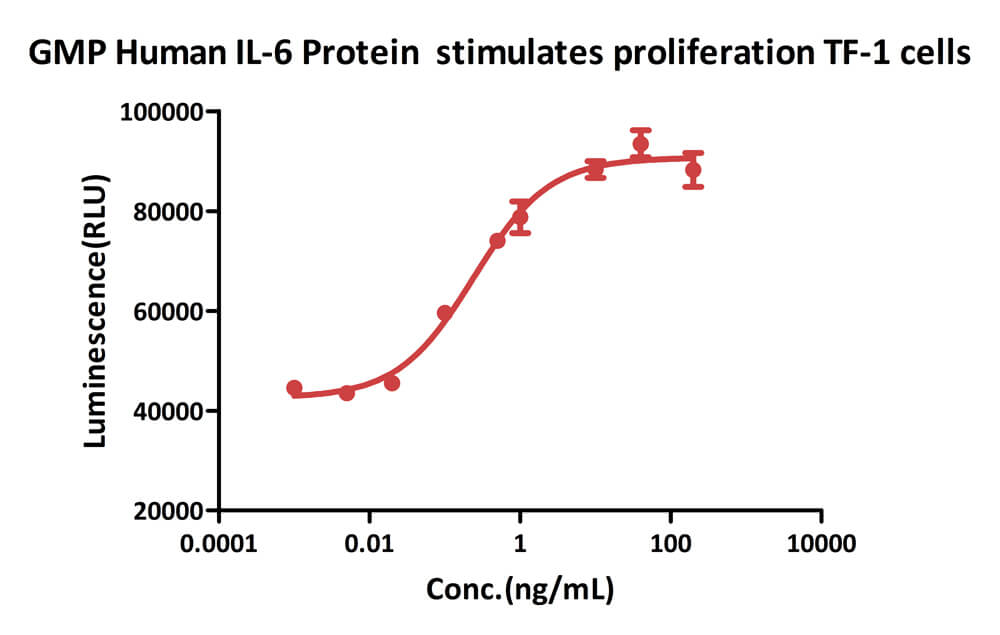
GMP Human IL-6 Protein (Cat. No. GMP-L06H27) stimulates proliferation of TF-1 human erythroleukemic cell line. The specific activity of GMP Human IL-6 Protein is > 1.00×10^8 IU/mg, which is calibrated against human IL-6 WHO International Standard (NIBSC code: 21/308) (QC tested).
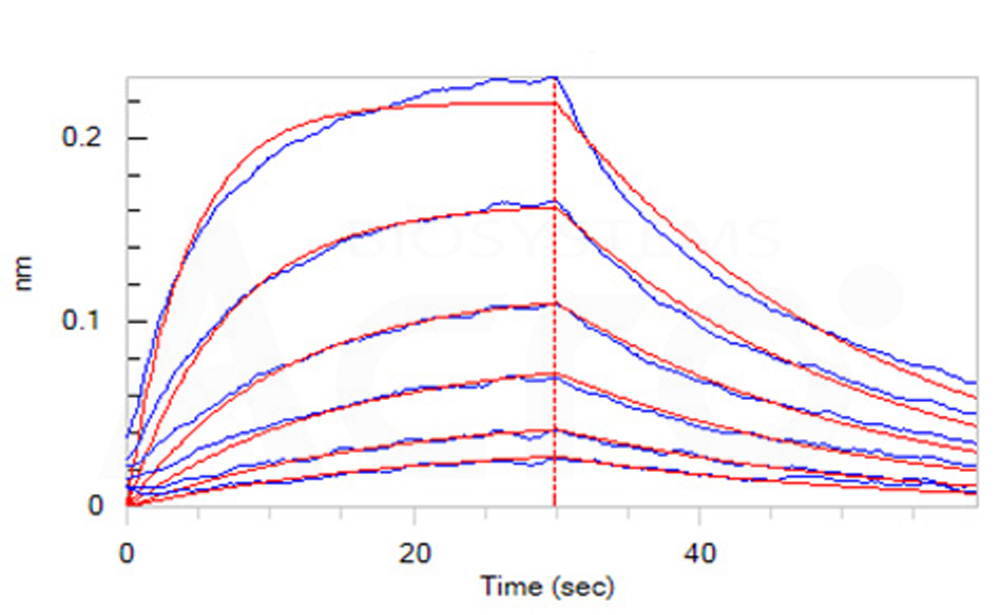
Loaded Biotinylated Human IL-6, epitope tag free, primary amine labeling (Cat. No. IL6-H8218) on SA Biosensor, can bind Human IL-6 R alpha, His Tag (Cat. No. ILR-H4223) with an affinity constant of 45.9 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Lenalidomide | IMID-5013; CDC-5013; CDC-501; CC-5013; IMiD-3; ENMD-0997; STAR-LLD | Approved | Celgene Corp | Revimid (former Brand Name), 瑞复美, Revlimid, Leavdo | United States | Myelodysplastic Syndromes | Bristol Myers Squibb Srlcompany | 2005-12-27 | Lymphoma, T-Cell, Peripheral; Optic Nerve Glioma; Intestinal Neoplasms; Immunoproliferative Small Intestinal Disease; Solid tumours; Bone Marrow Neoplasms; Lymphoma, B-Cell, Marginal Zone; Kidney Neoplasms; Leukemia; Liver Neoplasms; Hematologic Diseases; Leukemia, Erythroblastic, Acute; Leukemia, Promyelocytic, Acute; Leukemia, Myeloid; Ependymoma; HIV Infections; Ovarian Neoplasms; Medulloblastoma; Leukemia, Hairy Cell; Anemia; Paraproteinemias; Pain; Polycythemia Vera; Plaque, Amyloid; Rhabdoid Tumor; Anemia, Refractory, with Excess of Blasts; Glioblastoma; Smoldering Multiple Myeloma; Anemia, Refractory; Lymphoma, Large B-Cell, Diffuse; Hodgkin Disease; Myelodysplastic Syndromes; Hypothalamic Neoplasms; Neoplasms; Leukemia-Lymphoma, Adult T-Cell; Plasmacytoma; Blood Protein Disorders; Graft vs Host Disease; Leukemia, Myelomonocytic, Chronic; Leukemia, Myelomonocytic, Acute; Nerve Degeneration; Lymphomatoid Granulomatosis; Pancreatic Neoplasms; Multiple Myeloma; Leukemia, Megakaryoblastic, Acute; Oligodend | Details |
| Luminol sodium | MP-1032 | Approved | Selvim, Metrio | Immune System Diseases; Psoriasis | null | 1997-01-01 | Coronavirus Disease 2019 (COVID-19); Immune System Diseases; Psoriasis | Details | ||
| Pomalidomide | IMID-4047; CDC-394; CC-4047; IMiD-1 | Approved | Celgene Corp | Pomalyst, Imnovid, Actimid, Pomalyst/Imnovid, 安跃 | United States | Multiple Myeloma | Bristol-Myers Squibb Company | 2013-02-08 | Pulmonary Fibrosis; Atypical Squamous Cells of the Cervix; Sarcoma, Kaposi; Anemia, Sickle Cell; Neurofibromatosis 1; Lymphoma, Non-Hodgkin; Carcinoma, Small Cell; Waldenstrom Macroglobulinemia; Glioma; Primary Myelofibrosis; Sarcoma; Thrombocytosis; Prostatic Neoplasms; Lung Diseases, Interstitial; Medulloblastoma; Central Nervous System Neoplasms; Multiple Myeloma; Hodgkin Disease; Plasmacytoma; Kidney Diseases; Pancreatic Neoplasms; Immunoglobulin Light-chain Amyloidosis; Myeloproliferative Disorders; Graft vs Host Disease; Scleroderma, Systemic; Polycythemia Vera; Bone Marrow Neoplasms; Solid tumours | Details |
| Siltuximab | cCLB-8; CNTO-328 | Approved | Johnson & Johnson | Sylvant | United States | Multicentric Castleman's Disease (MCD) | Janssen Biotech Inc | 2014-04-23 | Psychotic Disorders; Leukemia, Large Granular Lymphocytic; Lymphoma, Non-Hodgkin; Lung Neoplasms; Thrombocytopenia; Colorectal Neoplasms; Castleman Disease; Primary Myelofibrosis; Prostatic Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Neoplasms, Plasma Cell; Multicentric Castleman's Disease (MCD); Lung Diseases; Monoclonal Gammopathy of Undetermined Significance; Respiratory Distress Syndrome, Adult; Multiple Myeloma; Bone Marrow Neoplasms; Immunoglobulin Light-chain Amyloidosis; Myelodysplastic Syndromes; Pancreatic Neoplasms; Smoldering Multiple Myeloma; Respiratory Tract Diseases; Schizophrenia; Pneumonia; Carcinoma, Renal Cell; Polycythemia Vera; Kidney Neoplasms; Respiratory Tract Infections; Cytokine Release Syndrome; Head and Neck Neoplasms; Ovarian Neoplasms; Diabetes Mellitus, Type 1 | Details |
| Andrographolide/Sodium Hydrogen Sulfite | Approved | Bacterial Infections | Details | |||||||
| Thalidomide | NSC-66847; NSC-527179; K-17; VP-02; FPF-300; FPF300 | Approved | Celgene Corp | Talizer, Thalidomide Celgene, Thalidomide Pharmion, Synovir, Thalomid, Thaled | Mainland China | Leprosy, Lepromatous; Multiple Myeloma | Changzhou Pharmaceutical Factory | 1982-01-01 | HIV Wasting Syndrome; Angiodysplasia; Primary Myelofibrosis; Neuroectodermal Tumors, Primitive; Prostatitis; Colorectal Neoplasms; Osteosarcoma; Lymphoma, Mantle-Cell; Sarcoma, Ewing; Retinoblastoma; Erythema Nodosum; Drug Resistant Epilepsy; Xerostomia; Sarcoma; Pancreatitis, Chronic; Adenocarcinoma, Clear Cell; Lymphoma, Follicular; Arachnoiditis; Carcinoma, Adenosquamous; Gastrointestinal Hemorrhage; Cholangitis, Sclerosing; Prostatic Neoplasms; Pelvic Pain; Neoplasm Metastasis; Stomatitis; Burning Mouth Syndrome; Mycobacterium avium-intracellulare Infection; Amyotrophic Lateral Sclerosis; Melanoma; Myelodysplastic-Myeloproliferative Diseases; Carcinoma, Hepatocellular; Leukemia, Lymphocytic, Chronic, B-Cell; Vascular Malformations; Tuberculosis; Appendiceal Neoplasms; Lymphoma, Non-Hodgkin; Uterine Neoplasms; Anemia, Sideroblastic; Glioma; Leprosy, Lepromatous; Endometrial Neoplasms; Lung Neoplasms; Waldenstrom Macroglobulinemia; Kidney Neoplasms; Thalassemia; Carcinoid Tumor; Lupus Erythematosus, Discoid | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Ensereptide | PXL-01 | Phase 3 Clinical | Promore Pharma | Post-surgical adhesions; Tissue Adhesions; Cicatrix | Details |
| Olokizumab | CDP-6038; Anti-IL6-UCB | Phase 3 Clinical | Ucb | Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19); Arthritis; Crohn Disease | Details |
| Efprezimod alfa | CD24-Fc; HAS-CD24; CD24-Fc-IgG; MK-7110; MK7110 | Phase 3 Clinical | Oncoimmune Inc | Solid tumours; Hematopoietic stem cell transplantation (HSCT); Leukemia; HIV Infections; Graft vs Host Disease; Myelodysplastic Syndromes; Coronavirus Disease 2019 (COVID-19); Precursor Cell Lymphoblastic Leukemia-Lymphoma; Dyslipidemias; Leukemia, Myeloid, Acute; Melanoma | Details |
| Ziltivekimab | COR-001 | Phase 3 Clinical | Astrazeneca Plc | Myocardial Infarction; Heart Failure; Anemia; Atherosclerosis; Cardiovascular Diseases; Inflammation; Renal Insufficiency, Chronic; Systemic Inflammatory Response Syndrome | Details |
| Clazakizumab | ALD-518; ALD518-003; BMS-645429; BMS-945429 | Phase 3 Clinical | Alder Biopharmaceuticals | Plaque, Atherosclerotic; Arthritis, Rheumatoid; Fatigue; Coronavirus Disease 2019 (COVID-19); Graft vs Host Disease; Rejection of organ transplantation; Arthritis, Psoriatic; Asthma; Cachexia; Crohn Disease; Carcinoma, Non-Small-Cell Lung; Kidney Failure, Chronic; Stomatitis | Details |
| RO-7200220 | RO-7200220 | Phase 3 Clinical | F. Hoffmann-La Roche Ltd | Macular Edema; Diabetic macular oedema | Details |
| PF-04236921 | PF-4236921; PF-04236921; TOUR006; TOUR-006 | Phase 2 Clinical | Pfizer Inc | Arthritis, Rheumatoid; Graves Ophthalmopathy; Lupus Erythematosus, Systemic; Renal Insufficiency, Chronic; Crohn Disease | Details |
| Sirukumab | BA-003; CNTO-136 | Phase 2 Clinical | Glaxosmithkline Plc, Johnson & Johnson Innovative Medicine | Giant Cell Arteritis; Depressive Disorder, Major; Arthritis, Rheumatoid; Lupus Erythematosus, Cutaneous; Coronavirus Disease 2019 (COVID-19); Lupus Nephritis; Polymyalgia Rheumatica; Lupus Erythematosus, Systemic; Asthma | Details |
| KSI-501 | KSI-501 | Phase 2 Clinical | Kodiak Sciences Inc | Retinal Diseases; Wet Macular Degeneration; Diabetic macular oedema; Uveitis | Details |
| Anti-interleukin-6 receptor monoclonal antibody (Biocad) | Phase 2 Clinical | Biocad | Autoimmune Diseases | Details | |
| Isomyosamine | MYMD-1 | Phase 2 Clinical | Mymd Pharmaceuticals Inc | Depression; Anxiety; Hashimoto Disease; Frailty; Coronavirus Disease 2019 (COVID-19); Arthritis, Rheumatoid; Healthy Aging; Inflammation; Sarcopenia | Details |
| FB-704A | FB-704; FB-704A | Phase 2 Clinical | Fountain Biopharma Inc | Arthritis, Rheumatoid; Asthma | Details |
| Gerilimzumab | GB-224; RYI-008; ARGX-109 | Phase 1 Clinical | Argenx Se | Arthritis, Rheumatoid | Details |
| MEDI-5117 | WBP-216; MEDI-5117 | Phase 1 Clinical | Wuxi Apptec Co Ltd, Medimmune | Arthritis, Rheumatoid | Details |
| Tocilizumab biosimilar (Mycenax Biotech) | Phase 1 Clinical | Mycenax Biotech Inc | Arthritis, Rheumatoid | Details | |
| NEX-20A | NEX-20A | Phase 1 Clinical | Nanexa AB | Multiple Myeloma | Details |
| Wogonin | Shandong Buchang Pharmaceuticals Co Ltd, Hefei Cosource Medicine Technology, Daewoong Pharmaceutical Co Ltd, China Pharmaceutical University | Details |
This web search service is supported by Google Inc.