 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| Cat. No. | Components | Size |
| GMP-CM3102 | CelThera™ GMP T Cell Expansion Medium (Phenol Red-free) | 1000mL |
| GMP-CM3101-1 | CelThera™ GMP T Cell Expansion Supplement | 7.25mL |

Compared to traditional culture media or xeno-free culture media, animal origin-free culture media can better reduce the risk of introducing potential pathogenic microorganisms during culture process, improve batch-to-batch consistency, and prevent T cell overactivation by undefined components in the serum.
CelThera™ GMP T Cell Expansion Medium (Phenol Red-free) does not require the addition of any serum or serum replacements and also maintains the high fold expansion of T cells. If users choose to add serum or serum replacement, the dosage should be determined by specific T cell applications.
The CelThera™ GMP T Cell Expansion Medium (Phenol Red-free) is stable for 18 months when stored under 2-8°C, protect from light.
The CelThera™ GMP T Cell Expansion Supplement is stable for 12 months when stored under -20°C or below, protect from light.
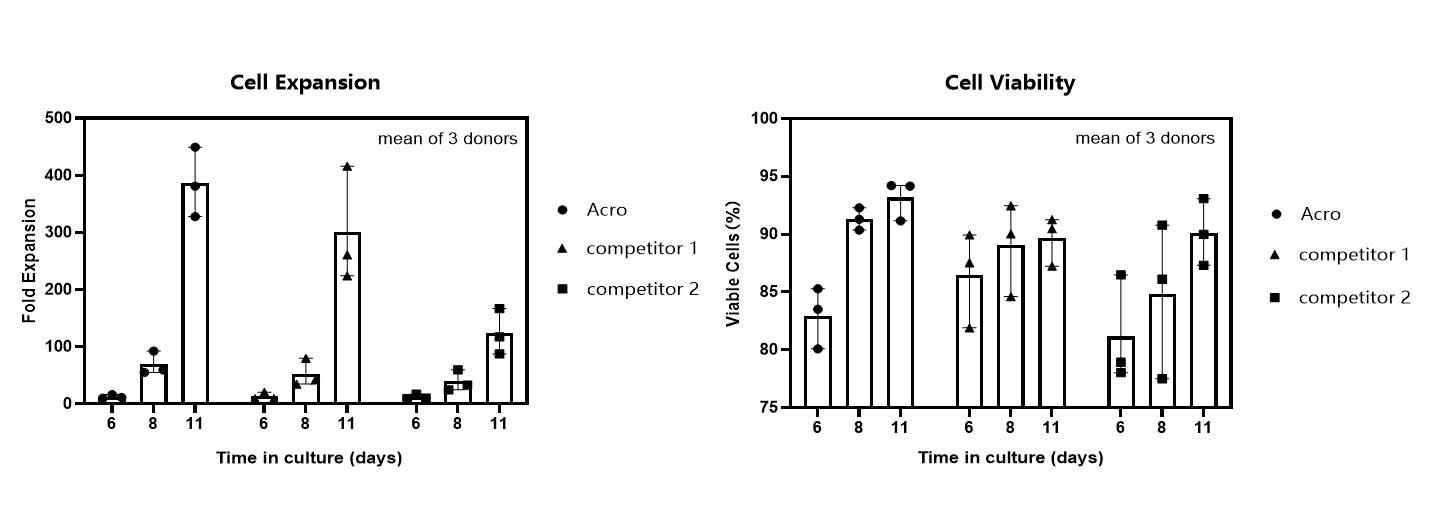
T cell expansion rate and cell viability in various media.
T cells from PBMCs of 3 different donors were activated and cultured for 11 days in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). Cell count and viability were performed on day 6, day 8 and day 11 by trypan blue staining. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3102) had a faster proliferation rate and higher viability than that of the other two media.
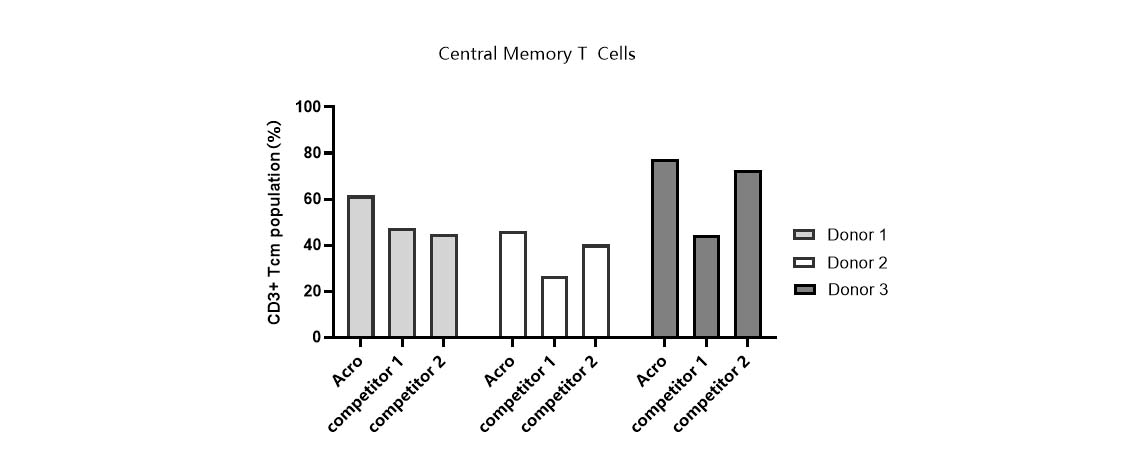
Tcm ratio in various media.
T cells from PBMCs of 3 different donors were activated and cultured in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). Tcm percentage (CD45RO+/CCR7+) was determined by flow cytometry when cells reached about 50-fold expansion. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3102) had a higher percentage of central memory T cells than that of the other two media.
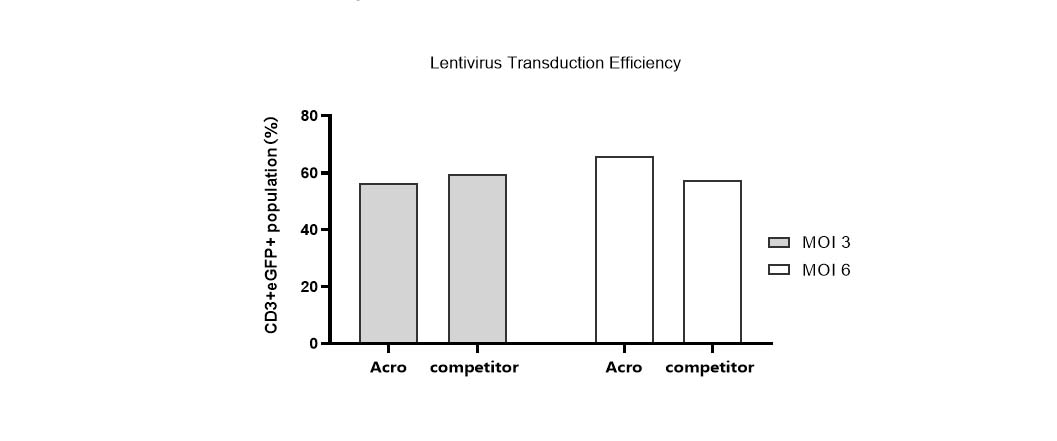
Lentivirus transduction efficiency in various media.
T cells from PBMCs were activated and cultured in various media supplemented with 300 IU/ml Acro GMP IL-2 (Cat. No. GMP-L02H14). 24 hours after activation, the cells were transduced with pLenti-CMV-EGFP-puro lentivirus (MOI=3 or 6). 24hrs after transduction, the lentivirus was removed by centrifugation. Then, the cells were cultured for 48hrs and the CD3+eGFP+ population was detected by flow cytometry. It indicated that cells in Acro T cell medium (Cat. No. GMP-CM3102) had a similar lentiviral transduction efficiency to that of the other medium.
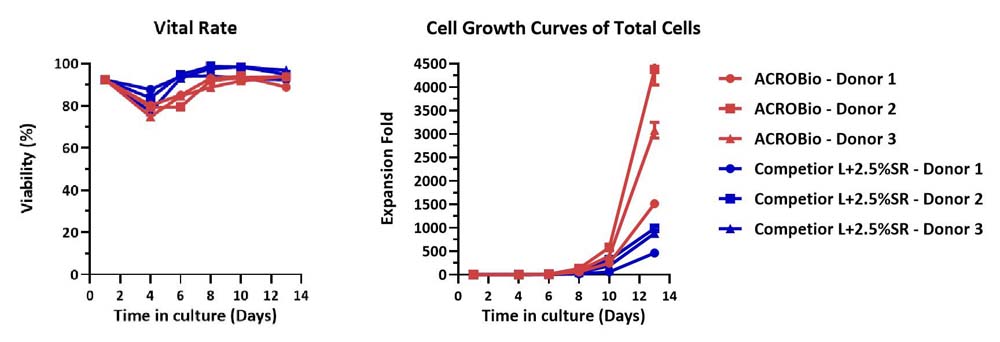
Human PBMCs were cultured with GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) with CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3102) or T cell culture medium (Competitor L +2.5% SR) for two weeks. The result shows that CelThera™ GMP T Cell Expansion Medium (ACROBiosystems) can be comparable to Competitor L +2.5% SR. Notably, the cells exhibit better expansion in CelThera™ GMP T Cell Expansion Medium (ACROBiosystems, Cat. No. GMP-CM3102).
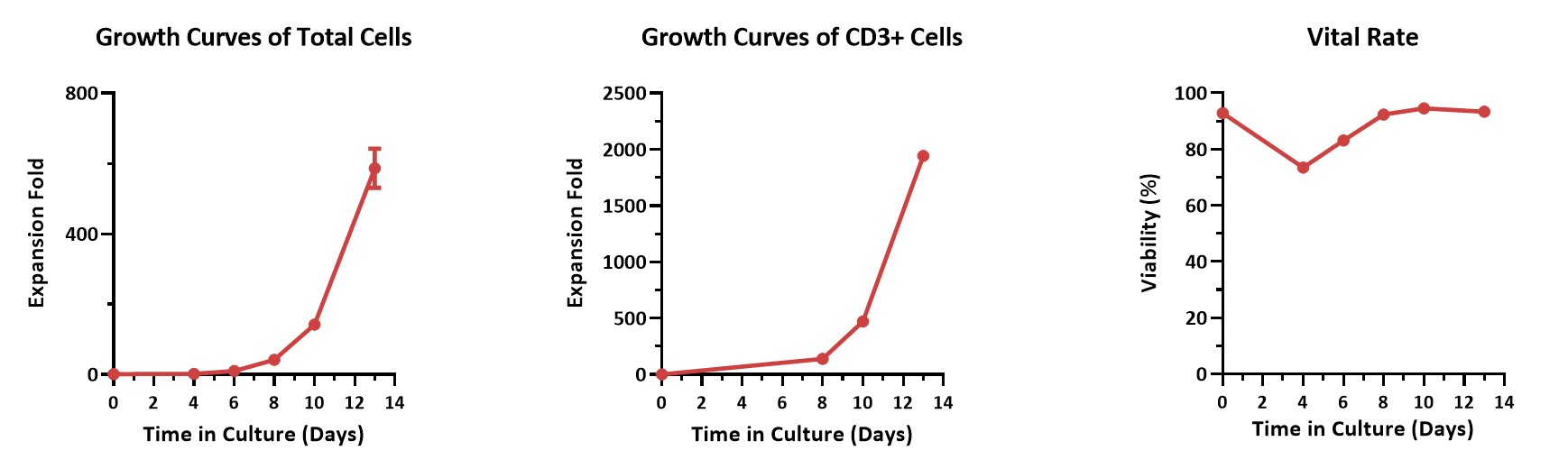
Human PBMCs were activated using 0.2 µg/mL GMP Monoclonal Anti-Human CD3 Antibody (OKT3) (ACROBiosystems, Cat. No. GMP-MC0323) and 1 µg/mL GMP Monoclonal Anti-Human CD28 Antibody (ACROBiosystems, Cat. No. GMP-MC2824), cultured with CelThera™ GMP T Cell Expansion culture medium (ACROBiosystems, Cat. No. GMP-CM3102) supplemented with 500 IU/mL GMP Human IL-2 Protein (ACROBiosystems, Cat. No. GMP-L02H14) for two weeks. The results showed that GMP human IL-2 protein, GMP monoclonal anti-human CD3 antibody (OKT3), GMP monoclonal anti-human CD28 antibody, and CelThrea™ GMP T cell expansion medium could be used to culture T cells in a 3L large system. It can efficiently expand cells with high viability.
ACROBiosystems GMP grade mediums are produced under a quality management system and in compliance with relevant guidelines: Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO 20399: 2022(E), Biotechnology — Ancillary materials present during the production of cellular therapeutic products and gene therapy products
ACROBiosystems Quality Management System Contents:
Designed and Manufactured under ISO 9001:2015 and ISO 13485:2016.
Animal-Free materials
Materials purchased from the approved suppliers by QA
ISO 5 clean room for filling
Qualified personnel
Quality-related documents review and approve by QA
Fully batch production and control records
Equipment maintenance and calibration
Validation of analytical procedures
Stability studies conducted
Comprehensive regulatory support files
Request For Regulatory Support Files(RSF)
ACROBiosystems provide rigorous quality control tests (fully validated equipment, processes and test methods) on our GMP grade products to ensure that they meet stringent standards in terms of purity, safety, activity and inter-batch stability, and each bulk QC lot mainly contains the following specific information:
pH
Sterility
Osmolality
Endotoxin Level
Functionality
Mycoplasma testing
Batch-to-batch consistency
ACROBiosystems GMP grade products are designed for research, manufacturing use or ex vivo use. CAUTION: Not intended for direct human use.
All products are warranted to meet ACROBiosystems Inc.’s (“ACRO”) published specifications when used under normal laboratory conditions.
ACRO DOES NOT MAKE ANY OTHER WARRANTY OR REPRESENTATION WHATSOEVER, WHETHER EXPRESS OR IMPLIED, WITH RESPECT TO ITS PRODUCTS. IN PARTICULAR, ACRO DOES NOT MAKE ANY WARRANTY OF SUITABILITY, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE.
NOT WITH STANDING ANY OTHER PROVISIONS OF THESE TERMS AND/OR ANY OTHER AGREEMENT BETWEEN ACRO AND PURCHASER FOR THE PURCAHSE OF THE PRODUCTS, ACRO’S TOTAL LIABILITY TO PURCHASER ARISING FROM OR IN RELATION TO THESE TERMS, AN AGREEMENT BETWEEN THE PARTIES OR THE PRODUCTS, WHETHER ARISING IN CONTRACT, TORT OR OTHERWISE SHALL BE LIMITED TO THE TOTAL AMOUNT PAID BY PURCHASER TO ACRO FOR THE RELEVANT PRODUCTS. IN NO EVENT WILL ACRO BE LIABLE FOR THE COST OF PROCUREMENT OF SUBSTITUTE GOODS.
The following terms are offered to you upon your acceptance of these End User Terms of Use of Product. By using this product, you indicate your acknowledgment and agreement to these End User Terms of Use of Product. If you do not agree to be bound by and comply with all of the provisions of these End User Terms of Use of Product, you should contact your supplier of the product and make arrangements to return the product.
The End User is aware that ACROBiosystems Inc. and its affiliate (“ACRO”) sell GMP grade products designed for research, manufacturing use or ex vivo use and not intended for human in vivo applications. The End User further agrees, as a condition of the sales of ACRO’s GMP grade products that: a) the End User will not use this GMP grade product in any procedure wherein the product may be directly or indirectly administered to humans, unless the End User has obtained, or prior to their use will have obtained, an Investigational New Drug (IND) exemption from the FDA and will use the product only in accordance with the protocols of such IND and of the Institutional Review Board overseeing the proposed research, or b) the End User will use the products outside of the United States in accordance with the protocols of research approved by the applicable review board or authorized ethics committee and regulatory agencies to which the End User is subject to in their territory.

ACROBiosystemsは、二重特異性抗体の治療薬への臨床開発を加速するために、高い生物活性を持つ均一なCD3δ/CD3εおよびCD3γ/CD3εタンパク質のシリーズを開発しました。

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox は、即使用可能のオルガノイド、オルガノイド分化ツールキット、創薬プロジェクトの進行を加速する、さまざまなサービスを含むオルガノイドソリューションのことです。

IL-15、IL-7、IL-21など、高品質のGMP準拠サイトカイン製品を提供しており、免疫細胞治療薬の臨床研究をサポートし、医薬品規制当局の承認を加速できます。

注目されている50以上のCAR-Tターゲットがあり、CARの発現の検出のために設計された抗原タンパク質をフローサイトメトリーで検証し、CARの発現が高い特異性で検出できます。ロット間の一貫性が保証されています。また、PE / FITC標識タンパク質を利用することで、ワンステップ染色でCARの発現を高い特異性でバックグラウンドなしに検出できます。

CD20、Claudin18.2、CD133、GPRC5D、CCR5、CCR8などの安定で高活性な全長型膜貫通タンパク質は、免疫学、ELISA、SPR、BLI、細胞実験、CAR陽性率検出に利用できます。VLP、界面活性剤、ナノディスクなどのテクニカルプラットフォームによって複数回膜貫通タンパク質の医薬品創薬を促進できます。

GMP準拠サイトカイン、高品質の細胞活性化/増幅試薬、遺伝子改変試薬/酵素、CAR検出試薬などの製品を提供し、細胞、遺伝子治療用に全体的なソリューションを提供し、創薬から臨床研究までのすべての段階をサポートします。

これまでに知られている免疫チェックポイント分子をほぼ製品化しており、様々なタグの製品群を提供できます。天然高分子の構造はMALSによって、生物活性はELISA/SPR/BLI/FACSなどによって検証済みです。またBiotin/FITCなどの標識も選択でき、抗体のハイスループットスクリーニングに利用できます。

弊社ACROBiosystemsはADC医薬品の開発のサポートに取り組み、注目されている様々なターゲットのために、異なる種類とタグの製品が開発され、高い純度と親和性が特長です。免疫、抗体スクリーン、SPR、細胞活性検査などの実験に利用できます。Protocolも無償で提供いたします。

すべての分子のFc受容体タンパク質だけでなく、一般的な変異体やビオチン標識タイプも含まれております。お客様のモノクローナル抗体の開発をサポートします。

インターロイキン、成長因子、ケモカイン、TNF など、様々なサイトカインターゲットの自然なコンフォメーションを確保するために HEK293 によって発現されます。SDS-PAGE/HPLC/SEC-MALS によって高純度は確認され、高い生物活性は ELISA/SPR/BLI によって確認されています。

AneuroはACROBiosystemsが神経科学研究のために設計した製品群のブランドです。神経科学研究を推進するための治療・診断用研究タンパク質、PFF、組み換え神経因子など、高品質の重要なタンパク質を提供しております。
This web search service is supported by Google Inc.