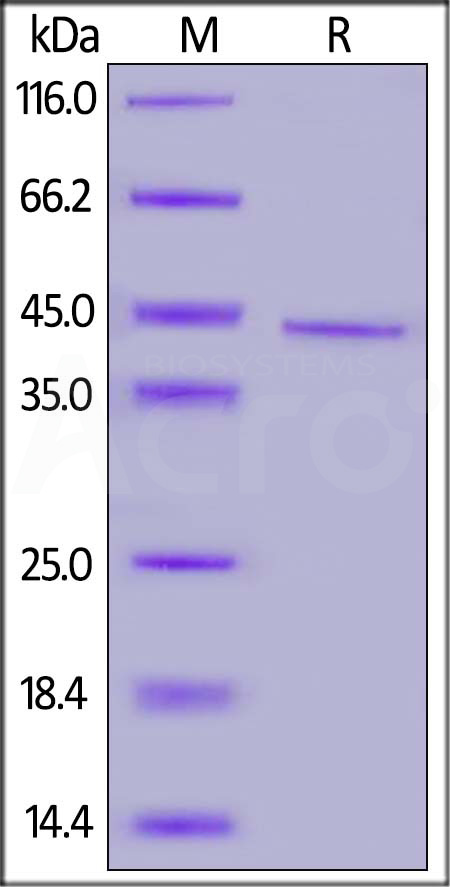
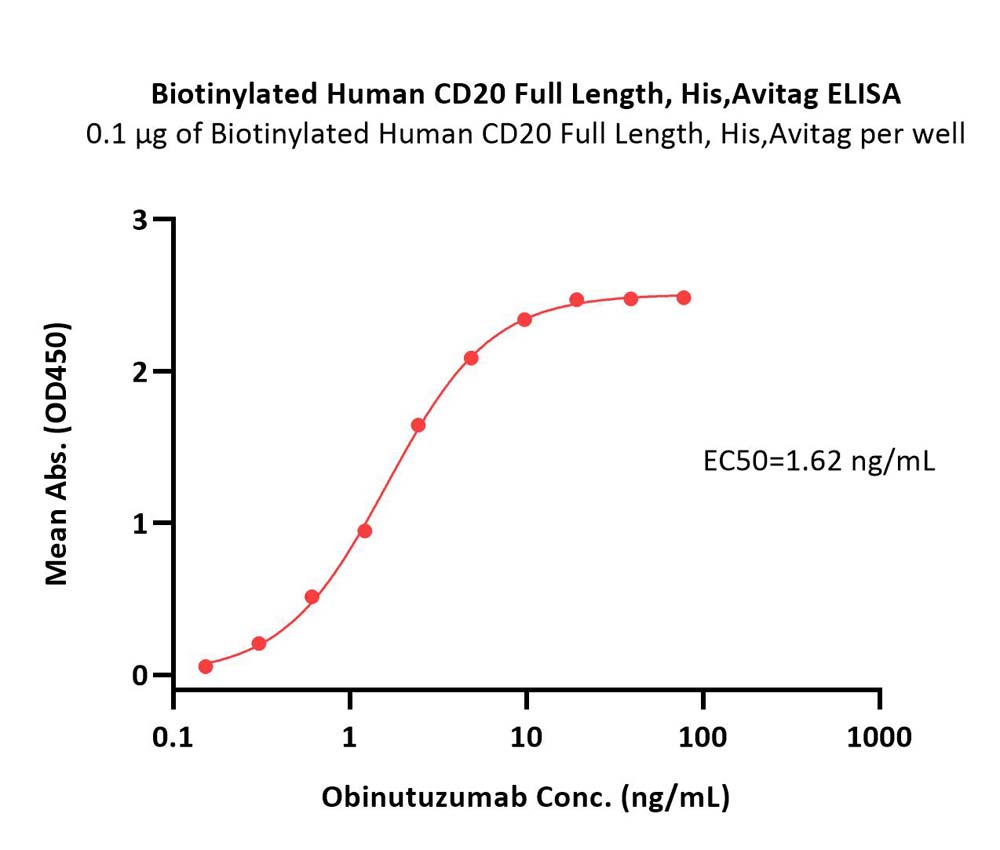
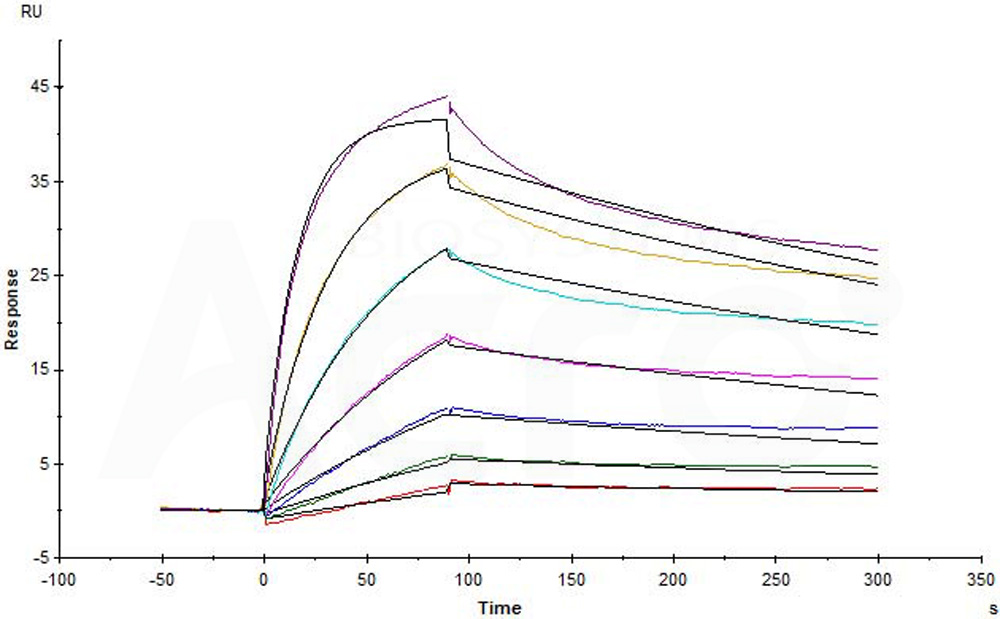
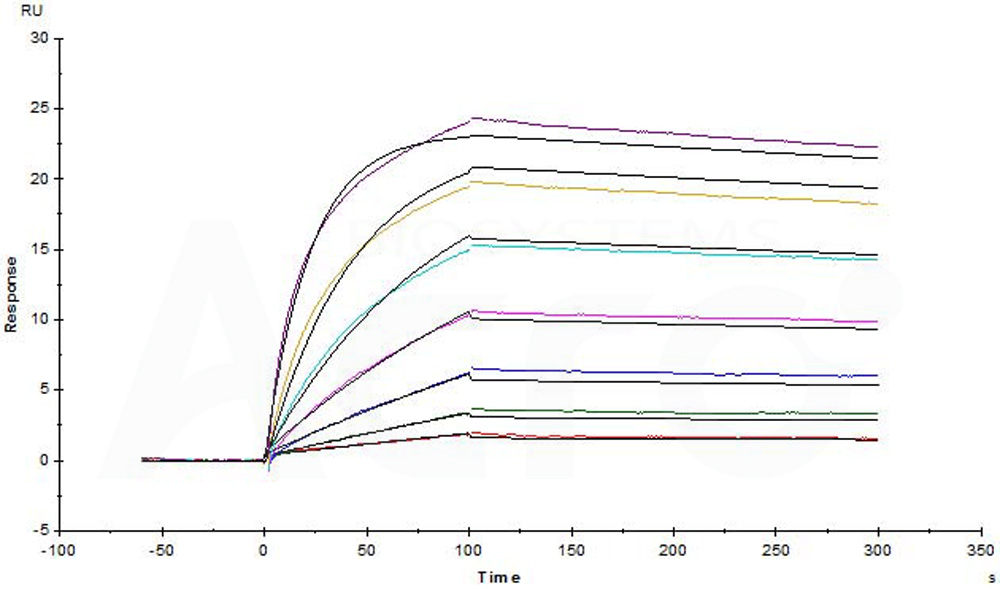
| Rituximab biosimilar (Samsung) |
SAIT-101 |
Phase 3 Clinical |
Samsung Biologics Co Ltd |
Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma; Lymphoma, T-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myelogenous, Chronic; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Arthritis, Rheumatoid; Anemia; Hematologic Neoplasms; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase |
Details
|
| Rituximab biosimilar (Hualan Biological Engineering) |
WBP-263 |
Phase 3 Clinical |
Hualan Genetic Engineering Co Ltd |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| Rituximab biosimilar (Boehringer Ingelheim) |
BI-695500 |
Phase 3 Clinical |
C.H. Boehringer Sohn Ag & Co. Kg |
Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Lymphoma; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Pancytopenia; Plasmablastic Lymphoma; Lymphoma, Mantle-Cell; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, B-Cell; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Leukemia, Myelomonocytic, Chronic; Arthritis, Rheumatoid; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Anemia; Candidiasis, Vulvovaginal; Hematologic Neoplasms; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic; Lymphoma, B-Cell, Marginal Zone |
Details
|
| Recombinant humanized monoclonal antibody MIL62 |
MIL62 |
Phase 3 Clinical |
Beijing Innocare Pharma Tech Co Ltd, Beijing Mabworks Biotech Co Ltd |
Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Nephrosis; Glomerulonephritis, Membranous; Lupus Nephritis; Lupus Erythematosus, Systemic; Lymphoma, Follicular; Neuromyelitis Optica; Lymphoma, Non-Hodgkin |
Details
|
| Recombinant chimeric anti-CD20 antibody (Shanghai Institute of Biological Products) |
|
Phase 3 Clinical |
Shanghai Institute Of Biological Products Co Ltd |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| Divozilimab |
BCD-132 |
Phase 3 Clinical |
Biocad |
Scleroderma, Systemic; Multiple Sclerosis; Neuromyelitis Optica |
Details
|
| Ocrelizumab biosimilar (CinnaGen) |
|
Phase 3 Clinical |
Cinnagen |
Multiple Sclerosis |
Details
|
| BAT-4406F |
BAT-4406; BAT-4406F |
Phase 3 Clinical |
Bio-Thera Solutions Ltd |
Neuromyelitis Optica |
Details
|
| Rituximab biosimilar (Nanjing Yoko Biomedical) |
GB-241 |
Phase 3 Clinical |
Genor Biopharma Co Ltd, Nanjing Yoko Biomedical Co Ltd |
Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma; Philadelphia Chromosome; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma, B-Cell; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Hematologic Neoplasms; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myelogenous, Chronic; Leukemia, Myeloid, Accelerated Phase |
Details
|
| Rituximab biosimilar (Hisun Pharma/Beijing Mabworks Biotech) |
|
Phase 3 Clinical |
Zhejiang Hisun Pharmaceutical Co Ltd, Beijing Mabworks Biotech Co Ltd |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| Ocrelizumab biosimilar (Celltrion) |
CT-P53 |
Phase 3 Clinical |
Celltrion Inc |
Multiple Sclerosis, Relapsing-Remitting |
Details
|
| Iodine 131 tositumomab |
TST/I131-TST |
Phase 3 Clinical |
Glaxosmithkline Plc |
Lymphoma, B-Cell; Leukemia, Lymphoid; Hodgkin Disease; Multiple Myeloma; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Recombinant anti-CD20 humanized monoclonal antibody (Shanghai Crosslink Pharmaceutical) |
B-007 |
Phase 3 Clinical |
Shanghai Crosslink Pharmaceutical R & D Co Ltd |
Myasthenia Gravis; Lymphoma, B-Cell; Nephrosis; Glomerulonephritis, Membranous; Lymphoma, Non-Hodgkin; Pemphigus |
Details
|
| Rituximab biosimilar(Shandong New Time Pharmaceutical) |
H-02; F-007 |
Phase 3 Clinical |
Shandong New Time Pharmaceutical Co Ltd |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| Imvotamab |
IGM-2323 |
Phase 2 Clinical |
Igm Biosciences Inc |
Lymphoma, B-Cell, Marginal Zone; Myositis; Lupus Erythematosus, Cutaneous; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Arthritis; Lymphoma, Non-Hodgkin |
Details
|
| Anti-CD20 CART-transduced T cells (Cellular Biomedicine Group) |
CART-20; CBM-C20.1; CBM-CD20 1 |
Phase 2 Clinical |
Pla General Hospital |
Leukemia; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Leukemia, Prolymphocytic |
Details
|
| BVX20-CD20 antibody (Biocon/Vaccinex) |
BVX-20; BVX20-MAb |
Phase 2 Clinical |
Biocon Ltd, Vaccinex Inc |
Lymphoma, Non-Hodgkin |
Details
|
| Dual specificity CD19 and CD20 or CD22 CAR-T cell therapy(Chinese PLA General Hospital) |
|
Phase 2 Clinical |
Pla General Hospital |
Lymphoma, B-Cell; Leukemia, B-Cell |
Details
|
| Anti-CD20 CAR T-cell therapy (Southwest Hospital Chongqing) |
|
Phase 2 Clinical |
Southwest Hospital Chongqing |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| Rituximab biosimilar (JHL Biotech) |
JHL-1101 |
Phase 2 Clinical |
JHL Biotech |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Blastic Plasmacytoid Dendritic Cell Neoplasm; Lymphoma, Follicular; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell |
Details
|
| Zamtocabtagene autoleucel |
MB-CART2019.1 |
Phase 2 Clinical |
Miltenyi Biotec |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| IMM-0306 |
IMC-002; IMM-0306 |
Phase 2 Clinical |
ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| Allogeneic anti-CD20 CAR-T cell therapy (Precision BioSciences) |
PBCAR-20A |
Phase 2 Clinical |
Precision Biosciences Inc |
Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| ELC-301 |
ELC301; ELC-301 |
Phase 2 Clinical |
Elicera Therapeutics AB, Uppsala University |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| MBS-303 |
MBS-303 |
Phase 2 Clinical |
Beijing Mabworks Biotech Co Ltd |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| UCART-20x22 |
UCART-20x22 |
Phase 2 Clinical |
Cellectis Sa |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| bbT-369 |
bbT-369 |
Phase 2 Clinical |
Bluebird Bio Inc |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| GB-261 |
GB-261 |
Phase 2 Clinical |
Genor Biopharma Co Ltd |
Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| CHO-H01 |
CHO-H01 |
Phase 2 Clinical |
Academia Sinica |
Neoplasms; Lymphoma, Non-Hodgkin |
Details
|
| MB-CART19.1 (Miltenyi Biotec) |
|
Phase 2 Clinical |
Miltenyi Biotec |
Lymphoma, B-Cell; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma |
Details
|
| 4SCAR-T cell therapy (Shenzhen Geno-Immune Medical Institute) |
4SCAR-T |
Phase 2 Clinical |
Shenzhen Geno-Immune Medical Institute |
Hematologic Neoplasms; Autoimmune Diseases; Neuroblastoma |
Details
|
| MRG001 |
MRG-001 |
Phase 2 Clinical |
|
Motor Neuron Disease; Lymphoma, B-Cell; Hepatitis, Alcoholic; Cytokine Release Syndrome; Respiratory Tract Diseases; Respiratory Distress Syndrome, Adult; Lymphoma, Non-Hodgkin; Respiratory Insufficiency; Amyotrophic Lateral Sclerosis |
Details
|
| IMPT-514 |
IMPT-514 |
Phase 2 Clinical |
ImmPACT Bio USA Inc |
Lupus Nephritis; Lupus Erythematosus, Systemic |
Details
|
| IPH-6501 |
IPH-6501; IPH-65 |
Phase 2 Clinical |
Innate Pharma SA |
Neoplasms; Lymphoma, Non-Hodgkin |
Details
|
| IMPT-314 |
IMPT-314 |
Phase 2 Clinical |
|
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| CD20 monoclonal antibody(The First Affiliated Hospital of Soochow University) |
|
Phase 2 Clinical |
The First Affiliated Hospital Of Soochow University |
Anemia, Aplastic |
Details
|
| CD19/CD20 bispecific CAR-T cells (Shanghai Cellular) |
|
Phase 2 Clinical |
Shanghai Cellular Biopharmaceutical Group Ltd |
Lymphoma, B-Cell |
Details
|
| ALETA-001 |
ALETA-001 |
Phase 2 Clinical |
Aleta BioTherapeutics Inc |
Lymphoma, B-Cell; Leukemia; Hematologic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin |
Details
|
| Rituximab Biosimilar (Istituto Giannina Gaslini) |
|
Phase 2 Clinical |
Istituto Giannina Gaslini |
Nephrotic Syndrome |
Details
|
| 1-A-46 |
BR-110; 1A46; 1-A-46; CMG1A46; CMG1A-46; BR110 |
Phase 2 Clinical |
Chengdu Chimagen Biosciences Co Ltd, BioRay Pharmaceutical Co Ltd |
Lymphoma, B-Cell; Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| ICP-B02 |
CM-355; ICP-B02 |
Phase 2 Clinical |
Beijing Tiannuo Jiancheng Pharmaceutical Technology Co Ltd, Keymed Biosciences Co Ltd |
Hematologic Neoplasms; Hematoma; Lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| CD19/CD20 dual-target CAR-T cell therapy (Shenzhen University General Hospital) |
|
Phase 2 Clinical |
Shenzhen University General Hospital |
Lymphoma, B-Cell |
Details
|
| Prizloncabtagene autoleucel |
C-CAR039; C-CAR-039; C CAR 039; EXP-039 |
Phase 2 Clinical |
Cellular Biomedicine Group Ltd |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| EX-103 |
EX-103; EX103 |
Phase 2 Clinical |
Guangzhou Excelmab Inc |
Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Anti-CD19 and anti-CD20 CAR-T cell therapy (Medical College of Wisconsin) |
|
Phase 2 Clinical |
The Medical College Of Wisconsin Nonprofit |
Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma |
Details
|
| Anti-CD20 CAR T-cell therapy (Shanghai Longyao Biotechnology) |
|
Phase 2 Clinical |
Shanghai Longyao Biological Technology Co Ltd |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| MB-106 |
MB-106 |
Phase 2 Clinical |
Fred Hutchinson Cancer Research Center |
Lymphoma, B-Cell; Lymphoma, Mantle-Cell; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Chimeric antigen receptor T cell therapeutics (targeted CD19/CD20/CD22/CD30,Shanghai Unicar-Therapy Bio-medicine) |
|
Phase 2 Clinical |
Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd |
Pancreatic Neoplasms; Lymphoma, Non-Hodgkin |
Details
|
| CD19/CD20 bispecific CAR-T cells (Henan Cancer Hospital) |
|
Phase 1 Clinical |
Henan Provincial Cancer Hospital |
Lymphoma, B-Cell |
Details
|
| Recombinant Fc-glycosylated anti-CD20 monoclonal antibody (Bio-Thera Solutions) |
BAT-4306F |
Phase 1 Clinical |
Bio-Thera Solutions Ltd |
Lymphoma, Non-Hodgkin |
Details
|
| TRS-005 |
TRS-005 |
Phase 1 Clinical |
Zhejiang Teruisi Pharmaceutical Inc |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| Rituximab biosimilar (International Biotech Center Generium) |
GNR-006 |
Phase 1 Clinical |
International Biotech Center Generium |
Lymphoma, B-Cell |
Details
|
| Recombinant anti-CD20 chimeric monoclonal antibody (Livzon Group) |
LZM-002 |
Phase 1 Clinical |
Livzon Pharmaceutical Group Inc |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin |
Details
|
| MB-CART20.1 (Miltenyi Biotec) |
|
Phase 1 Clinical |
Miltenyi Biotec |
Melanoma |
Details
|
| Humanized anti-CD19 and anti-CD20 dual specific CAR-T cell therapy (Fujian Medical University) |
|
Phase 1 Clinical |
Fujian Medical University |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| Anti-CD20 B9E9 scFv-Streptavidin Fusion Protein (Fred Hutchinson Cancer Research Center) |
|
Phase 1 Clinical |
Fred Hutchinson Cancer Research Center, National Cancer Institute |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| Rituximab biosimilar (Shanghai CP Guojian Pharmaceutical) |
CMAB-304; 304R |
Phase 1 Clinical |
Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| Rituximab biosimilar (Fred Hutchinson Cancer Research Center) |
|
Phase 1 Clinical |
Fred Hutchinson Cancer Research Center |
Lymphoma, Large B-Cell, Diffuse; Multicentric Castleman's Disease (MCD); Burkitt Lymphoma; Sarcoma, Kaposi |
Details
|
| Anti-CD20 allogeneic CAR-T cell therapy (Nanjing Medical University) |
LUCAR-20S |
Phase 1 Clinical |
|
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Bendamustine Hydrochloride/Rituximab |
|
Phase 1 Clinical |
1globe Biomedical Co Ltd |
Lymphoma, B-Cell |
Details
|
| Recombinant anti-CD20 humanized monoclonal antibody (Shanghai Pharma) |
B001; B-001; B001A; B001-A; B001C; B001-C |
Phase 1 Clinical |
Shanghai Pharmaceuticals Holding Co Ltd |
Multiple Sclerosis; Neuromyelitis Optica; Lymphoma, Non-Hodgkin; Lymphoma |
Details
|
| Rituximab biosimilar (Gedeon Richter) |
RGB-03 |
Phase 1 Clinical |
Gedeon Richter |
Arthritis, Rheumatoid |
Details
|
| CD20-directed CAR-T cell therapy (Tongji Hospital) |
C-CAR066 |
Phase 1 Clinical |
Tongji Hospital, Cellular Biomedicine Group Inc, Shanghai Cellular Biopharmaceutical Group Ltd |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| JS-203 |
JS-203 |
Phase 1 Clinical |
Shanghai Junshi Biological Engineering Co Ltd |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| Dual CD19/CD20 targeting CAR-T therapy(Poseida Therapeutics) |
|
Phase 1 Clinical |
Poseida Therapeutics Inc |
Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| CD20 CAR-T cell therapy (Shanghai Longyao Biotechnology) |
|
Phase 1 Clinical |
Shanghai Longyao Biological Technology Co Ltd |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| KITE-363 |
KITE-363 |
Phase 1 Clinical |
|
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse |
Details
|
| ACE-1831 |
ACE-1831 |
Phase 1 Clinical |
Acepodia Biotech Inc |
Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Primary mediastinal B cell lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| CD19/CD20 bispecific chimeric antigen receptor (CAR)-T cell therapy (Jonsson Comprehensive Cancer Center) |
|
Phase 1 Clinical |
Uclas Jonsson Comprehensive Cancer Center |
Candidiasis, Vulvovaginal; Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| LUCAR-G39P |
LUCAR-G39P |
Phase 1 Clinical |
|
Lymphoma, Non-Hodgkin |
Details
|
| Allogenic Chimeric Antigen Receptor(CAR)-T Cell Therapy(Beijing Cancer Hospital) |
LUCAR-20SP |
Phase 1 Clinical |
Beijing Cancer Hospital |
Lymphoma, Non-Hodgkin |
Details
|
| Rituximab biosimilar (Mabscale) |
|
Phase 1 Clinical |
Mabscale LLC |
Arthritis, Rheumatoid |
Details
|
| ASP-2802 |
ASP2802; ASP-2802 |
Phase 1 Clinical |
Astellas Pharma Global Development Inc |
Lymphoma, B-Cell |
Details
|
| JNJ-1493 |
JNJ-1493 |
Phase 1 Clinical |
Xencor Inc |
Hematologic Neoplasms; Lymphoma, B-Cell |
Details
|
| C-CAR168 CAR T-cell therapy (AbelZeta) |
C-CAR168 |
Phase 1 Clinical |
AbelZeta Pharma Inc |
Multiple Sclerosis, Relapsing-Remitting; Autoimmune Diseases; Neuromyelitis Optica; Lupus Erythematosus, Systemic; Muscular Diseases |
Details
|
| SCTB-35 |
SCTB35; SCTB-35 |
Phase 1 Clinical |
SinoCelltech Ltd |
Lymphoma, B-Cell |
Details
|
| Recombinant human anti-CD20 momoclonal antibody(Shanghai Crosslink Pharma) |
|
Phase 1 Clinical |
Shanghai Crosslink Pharmaceutical R & D Co Ltd |
Myasthenia Gravis; Pemphigus |
Details
|
| Anti-CD19 and Anti-CD20 Bicistronic Chimeric Antigen Receptor T Cells(NIH) |
|
Phase 1 Clinical |
National Cancer Institute |
Lymphoma, B-Cell; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| RO-7121932 |
RO7121932; RO-7121932; RO 7121932; RG-6035 |
Phase 1 Clinical |
Genentech Inc |
Multiple Sclerosis |
Details
|
| TanCART19/20 |
|
Phase 1 Clinical |
Pla General Hospital |
Neuromyelitis Optica |
Details
|
| LCAR-AIO |
LCAR-AIO; VHH CAR-T |
Phase 1 Clinical |
|
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma |
Details
|
| CAR-20-19-22 |
CAR-20-19-22 |
Phase 1 Clinical |
The Medical College Of Wisconsin Nonprofit |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Rituximab biosimilar (Zhejiang Teruisi Pharmaceutical) |
TRS001 |
Phase 1 Clinical |
Zhejiang Teruisi Pharmaceutical Inc |
Lymphoma |
Details
|
| QLP-31907 |
QLP-31907; QLP31907; PSB-202 |
Phase 1 Clinical |
Qilu Pharmaceutical Co Ltd |
Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| ADI-001 |
ADI-001 |
Phase 1 Clinical |
|
Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Primary mediastinal B cell lymphoma; Lymphoma, Non-Hodgkin; Burkitt Lymphoma |
Details
|
| TQB-2825 |
TQB-2825 |
Phase 1 Clinical |
Wuxi Biologics Co Ltd |
Hematologic Neoplasms |
Details
|
| CD19/CD20 CAR-T Cell Therapy (PersonGen) |
|
Phase 1 Clinical |
Persongen Biotherapeutics |
|
Details
|
| JMT-601 |
JMT-601 |
Phase 1 Clinical |
Shanghai Jmt-Bio Inc |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| LY007 |
LY007 |
Phase 1 Clinical |
Shanghai Longyao Biological Technology Co Ltd |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin |
Details
|
| Autologous humanized anti-CD19 and anti-CD20 dual specific CAR-T cell therapy (First Affiliated Hospital of Zhejiang University) |
|
Phase 1 Clinical |
First Affiliated Hospital Of Zhejiang University |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| Plamotamab |
XmAb-13676 |
Phase 1 Clinical |
Xencor Inc |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| CD20/CD22 dual Targeted CAR T-cell therapy (Zhejiang University) |
|
Phase 1 Clinical |
Zhejiang University, Shanghai YaKe Biotechnology Co Ltd |
Hematologic Neoplasms |
Details
|
| Rituximab/Paclitaxel |
AR-160 |
Phase 1 Clinical |
Mayo Clinic |
Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Anti-CD20 CAR-T cell therapy (Wuhan Bio-Raid) |
|
Clinical |
Wuhan BioRaid Biotechnology Co Ltd |
Neoplasms |
Details
|
| Rituximab biosimilar(Bioxpress) |
BXT-2336 |
Clinical |
Bioxpress Therapeutics Sa |
Granulomatosis with Polyangiitis; Microscopic Polyangiitis; Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Rituximab/Bendamustine/Cytarabine |
|
|
Fondazione Italiana Linfomi Onlus |
|
Details
|
| Rituximab biosimilar (Samsung) |
SAIT-101 |
Phase 3 Clinical |
Samsung Biologics Co Ltd |
Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma; Lymphoma, T-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myelogenous, Chronic; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Arthritis, Rheumatoid; Anemia; Hematologic Neoplasms; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myeloid, Accelerated Phase |
Details
|
| Rituximab biosimilar (Hualan Biological Engineering) |
WBP-263 |
Phase 3 Clinical |
Hualan Genetic Engineering Co Ltd |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| Rituximab biosimilar (Boehringer Ingelheim) |
BI-695500 |
Phase 3 Clinical |
C.H. Boehringer Sohn Ag & Co. Kg |
Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Lymphoma; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, B-Cell; Lymphoproliferative Disorders; Philadelphia Chromosome; Pancytopenia; Plasmablastic Lymphoma; Lymphoma, Mantle-Cell; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, B-Cell; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Leukemia, Myelomonocytic, Chronic; Arthritis, Rheumatoid; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Anemia; Candidiasis, Vulvovaginal; Hematologic Neoplasms; Leukemia, Myeloid, Accelerated Phase; Leukemia, Myelogenous, Chronic; Lymphoma, B-Cell, Marginal Zone |
Details
|
| Recombinant humanized monoclonal antibody MIL62 |
MIL62 |
Phase 3 Clinical |
Beijing Innocare Pharma Tech Co Ltd, Beijing Mabworks Biotech Co Ltd |
Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Nephrosis; Glomerulonephritis, Membranous; Lupus Nephritis; Lupus Erythematosus, Systemic; Lymphoma, Follicular; Neuromyelitis Optica; Lymphoma, Non-Hodgkin |
Details
|
| Recombinant chimeric anti-CD20 antibody (Shanghai Institute of Biological Products) |
|
Phase 3 Clinical |
Shanghai Institute Of Biological Products Co Ltd |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| Divozilimab |
BCD-132 |
Phase 3 Clinical |
Biocad |
Scleroderma, Systemic; Multiple Sclerosis; Neuromyelitis Optica |
Details
|
| Ocrelizumab biosimilar (CinnaGen) |
|
Phase 3 Clinical |
Cinnagen |
Multiple Sclerosis |
Details
|
| BAT-4406F |
BAT-4406; BAT-4406F |
Phase 3 Clinical |
Bio-Thera Solutions Ltd |
Neuromyelitis Optica |
Details
|
| Rituximab biosimilar (Nanjing Yoko Biomedical) |
GB-241 |
Phase 3 Clinical |
Genor Biopharma Co Ltd, Nanjing Yoko Biomedical Co Ltd |
Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell; Lymphoma, T-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma; Philadelphia Chromosome; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma, B-Cell; Blastic Plasmacytoid Dendritic Cell Neoplasm; Blast Crisis; Neoplasms; Lymphoma, Large B-Cell, Diffuse; Hematologic Neoplasms; Lymphoma, B-Cell, Marginal Zone; Leukemia, Myelogenous, Chronic; Leukemia, Myeloid, Accelerated Phase |
Details
|
| Rituximab biosimilar (Hisun Pharma/Beijing Mabworks Biotech) |
|
Phase 3 Clinical |
Zhejiang Hisun Pharmaceutical Co Ltd, Beijing Mabworks Biotech Co Ltd |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| Ocrelizumab biosimilar (Celltrion) |
CT-P53 |
Phase 3 Clinical |
Celltrion Inc |
Multiple Sclerosis, Relapsing-Remitting |
Details
|
| Iodine 131 tositumomab |
TST/I131-TST |
Phase 3 Clinical |
Glaxosmithkline Plc |
Lymphoma, B-Cell; Leukemia, Lymphoid; Hodgkin Disease; Multiple Myeloma; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Recombinant anti-CD20 humanized monoclonal antibody (Shanghai Crosslink Pharmaceutical) |
B-007 |
Phase 3 Clinical |
Shanghai Crosslink Pharmaceutical R & D Co Ltd |
Myasthenia Gravis; Lymphoma, B-Cell; Nephrosis; Glomerulonephritis, Membranous; Lymphoma, Non-Hodgkin; Pemphigus |
Details
|
| Rituximab biosimilar(Shandong New Time Pharmaceutical) |
H-02; F-007 |
Phase 3 Clinical |
Shandong New Time Pharmaceutical Co Ltd |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| Imvotamab |
IGM-2323 |
Phase 2 Clinical |
Igm Biosciences Inc |
Lymphoma, B-Cell, Marginal Zone; Myositis; Lupus Erythematosus, Cutaneous; Arthritis, Rheumatoid; Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Arthritis; Lymphoma, Non-Hodgkin |
Details
|
| Anti-CD20 CART-transduced T cells (Cellular Biomedicine Group) |
CART-20; CBM-C20.1; CBM-CD20 1 |
Phase 2 Clinical |
Pla General Hospital |
Leukemia; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Leukemia, Prolymphocytic |
Details
|
| BVX20-CD20 antibody (Biocon/Vaccinex) |
BVX-20; BVX20-MAb |
Phase 2 Clinical |
Biocon Ltd, Vaccinex Inc |
Lymphoma, Non-Hodgkin |
Details
|
| Dual specificity CD19 and CD20 or CD22 CAR-T cell therapy(Chinese PLA General Hospital) |
|
Phase 2 Clinical |
Pla General Hospital |
Lymphoma, B-Cell; Leukemia, B-Cell |
Details
|
| Anti-CD20 CAR T-cell therapy (Southwest Hospital Chongqing) |
|
Phase 2 Clinical |
Southwest Hospital Chongqing |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| Rituximab biosimilar (JHL Biotech) |
JHL-1101 |
Phase 2 Clinical |
JHL Biotech |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Arthritis, Rheumatoid; Blastic Plasmacytoid Dendritic Cell Neoplasm; Lymphoma, Follicular; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell |
Details
|
| Zamtocabtagene autoleucel |
MB-CART2019.1 |
Phase 2 Clinical |
Miltenyi Biotec |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| IMM-0306 |
IMC-002; IMM-0306 |
Phase 2 Clinical |
ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| Allogeneic anti-CD20 CAR-T cell therapy (Precision BioSciences) |
PBCAR-20A |
Phase 2 Clinical |
Precision Biosciences Inc |
Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| ELC-301 |
ELC301; ELC-301 |
Phase 2 Clinical |
Elicera Therapeutics AB, Uppsala University |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| MBS-303 |
MBS-303 |
Phase 2 Clinical |
Beijing Mabworks Biotech Co Ltd |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| UCART-20x22 |
UCART-20x22 |
Phase 2 Clinical |
Cellectis Sa |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| bbT-369 |
bbT-369 |
Phase 2 Clinical |
Bluebird Bio Inc |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| GB-261 |
GB-261 |
Phase 2 Clinical |
Genor Biopharma Co Ltd |
Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| CHO-H01 |
CHO-H01 |
Phase 2 Clinical |
Academia Sinica |
Neoplasms; Lymphoma, Non-Hodgkin |
Details
|
| MB-CART19.1 (Miltenyi Biotec) |
|
Phase 2 Clinical |
Miltenyi Biotec |
Lymphoma, B-Cell; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma |
Details
|
| 4SCAR-T cell therapy (Shenzhen Geno-Immune Medical Institute) |
4SCAR-T |
Phase 2 Clinical |
Shenzhen Geno-Immune Medical Institute |
Hematologic Neoplasms; Autoimmune Diseases; Neuroblastoma |
Details
|
| MRG001 |
MRG-001 |
Phase 2 Clinical |
|
Motor Neuron Disease; Lymphoma, B-Cell; Hepatitis, Alcoholic; Cytokine Release Syndrome; Respiratory Tract Diseases; Respiratory Distress Syndrome, Adult; Lymphoma, Non-Hodgkin; Respiratory Insufficiency; Amyotrophic Lateral Sclerosis |
Details
|
| IMPT-514 |
IMPT-514 |
Phase 2 Clinical |
ImmPACT Bio USA Inc |
Lupus Nephritis; Lupus Erythematosus, Systemic |
Details
|
| IPH-6501 |
IPH-6501; IPH-65 |
Phase 2 Clinical |
Innate Pharma SA |
Neoplasms; Lymphoma, Non-Hodgkin |
Details
|
| IMPT-314 |
IMPT-314 |
Phase 2 Clinical |
|
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| CD20 monoclonal antibody(The First Affiliated Hospital of Soochow University) |
|
Phase 2 Clinical |
The First Affiliated Hospital Of Soochow University |
Anemia, Aplastic |
Details
|
| CD19/CD20 bispecific CAR-T cells (Shanghai Cellular) |
|
Phase 2 Clinical |
Shanghai Cellular Biopharmaceutical Group Ltd |
Lymphoma, B-Cell |
Details
|
| ALETA-001 |
ALETA-001 |
Phase 2 Clinical |
Aleta BioTherapeutics Inc |
Lymphoma, B-Cell; Leukemia; Hematologic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin |
Details
|
| Rituximab Biosimilar (Istituto Giannina Gaslini) |
|
Phase 2 Clinical |
Istituto Giannina Gaslini |
Nephrotic Syndrome |
Details
|
| 1-A-46 |
BR-110; 1A46; 1-A-46; CMG1A46; CMG1A-46; BR110 |
Phase 2 Clinical |
Chengdu Chimagen Biosciences Co Ltd, BioRay Pharmaceutical Co Ltd |
Lymphoma, B-Cell; Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| ICP-B02 |
CM-355; ICP-B02 |
Phase 2 Clinical |
Beijing Tiannuo Jiancheng Pharmaceutical Technology Co Ltd, Keymed Biosciences Co Ltd |
Hematologic Neoplasms; Hematoma; Lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| CD19/CD20 dual-target CAR-T cell therapy (Shenzhen University General Hospital) |
|
Phase 2 Clinical |
Shenzhen University General Hospital |
Lymphoma, B-Cell |
Details
|
| Prizloncabtagene autoleucel |
C-CAR039; C-CAR-039; C CAR 039; EXP-039 |
Phase 2 Clinical |
Cellular Biomedicine Group Ltd |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| EX-103 |
EX-103; EX103 |
Phase 2 Clinical |
Guangzhou Excelmab Inc |
Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Anti-CD19 and anti-CD20 CAR-T cell therapy (Medical College of Wisconsin) |
|
Phase 2 Clinical |
The Medical College Of Wisconsin Nonprofit |
Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma |
Details
|
| Anti-CD20 CAR T-cell therapy (Shanghai Longyao Biotechnology) |
|
Phase 2 Clinical |
Shanghai Longyao Biological Technology Co Ltd |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| MB-106 |
MB-106 |
Phase 2 Clinical |
Fred Hutchinson Cancer Research Center |
Lymphoma, B-Cell; Lymphoma, Mantle-Cell; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Chimeric antigen receptor T cell therapeutics (targeted CD19/CD20/CD22/CD30,Shanghai Unicar-Therapy Bio-medicine) |
|
Phase 2 Clinical |
Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd |
Pancreatic Neoplasms; Lymphoma, Non-Hodgkin |
Details
|
| CD19/CD20 bispecific CAR-T cells (Henan Cancer Hospital) |
|
Phase 1 Clinical |
Henan Provincial Cancer Hospital |
Lymphoma, B-Cell |
Details
|
| Recombinant Fc-glycosylated anti-CD20 monoclonal antibody (Bio-Thera Solutions) |
BAT-4306F |
Phase 1 Clinical |
Bio-Thera Solutions Ltd |
Lymphoma, Non-Hodgkin |
Details
|
| TRS-005 |
TRS-005 |
Phase 1 Clinical |
Zhejiang Teruisi Pharmaceutical Inc |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| Rituximab biosimilar (International Biotech Center Generium) |
GNR-006 |
Phase 1 Clinical |
International Biotech Center Generium |
Lymphoma, B-Cell |
Details
|
| Recombinant anti-CD20 chimeric monoclonal antibody (Livzon Group) |
LZM-002 |
Phase 1 Clinical |
Livzon Pharmaceutical Group Inc |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin |
Details
|
| MB-CART20.1 (Miltenyi Biotec) |
|
Phase 1 Clinical |
Miltenyi Biotec |
Melanoma |
Details
|
| Humanized anti-CD19 and anti-CD20 dual specific CAR-T cell therapy (Fujian Medical University) |
|
Phase 1 Clinical |
Fujian Medical University |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| Anti-CD20 B9E9 scFv-Streptavidin Fusion Protein (Fred Hutchinson Cancer Research Center) |
|
Phase 1 Clinical |
Fred Hutchinson Cancer Research Center, National Cancer Institute |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| Rituximab biosimilar (Shanghai CP Guojian Pharmaceutical) |
CMAB-304; 304R |
Phase 1 Clinical |
Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd |
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| Rituximab biosimilar (Fred Hutchinson Cancer Research Center) |
|
Phase 1 Clinical |
Fred Hutchinson Cancer Research Center |
Lymphoma, Large B-Cell, Diffuse; Multicentric Castleman's Disease (MCD); Burkitt Lymphoma; Sarcoma, Kaposi |
Details
|
| Anti-CD20 allogeneic CAR-T cell therapy (Nanjing Medical University) |
LUCAR-20S |
Phase 1 Clinical |
|
Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Bendamustine Hydrochloride/Rituximab |
|
Phase 1 Clinical |
1globe Biomedical Co Ltd |
Lymphoma, B-Cell |
Details
|
| Recombinant anti-CD20 humanized monoclonal antibody (Shanghai Pharma) |
B001; B-001; B001A; B001-A; B001C; B001-C |
Phase 1 Clinical |
Shanghai Pharmaceuticals Holding Co Ltd |
Multiple Sclerosis; Neuromyelitis Optica; Lymphoma, Non-Hodgkin; Lymphoma |
Details
|
| Rituximab biosimilar (Gedeon Richter) |
RGB-03 |
Phase 1 Clinical |
Gedeon Richter |
Arthritis, Rheumatoid |
Details
|
| CD20-directed CAR-T cell therapy (Tongji Hospital) |
C-CAR066 |
Phase 1 Clinical |
Tongji Hospital, Cellular Biomedicine Group Inc, Shanghai Cellular Biopharmaceutical Group Ltd |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| JS-203 |
JS-203 |
Phase 1 Clinical |
Shanghai Junshi Biological Engineering Co Ltd |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| Dual CD19/CD20 targeting CAR-T therapy(Poseida Therapeutics) |
|
Phase 1 Clinical |
Poseida Therapeutics Inc |
Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| CD20 CAR-T cell therapy (Shanghai Longyao Biotechnology) |
|
Phase 1 Clinical |
Shanghai Longyao Biological Technology Co Ltd |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin |
Details
|
| KITE-363 |
KITE-363 |
Phase 1 Clinical |
|
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse |
Details
|
| ACE-1831 |
ACE-1831 |
Phase 1 Clinical |
Acepodia Biotech Inc |
Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Primary mediastinal B cell lymphoma; Lymphoma, Non-Hodgkin |
Details
|
| CD19/CD20 bispecific chimeric antigen receptor (CAR)-T cell therapy (Jonsson Comprehensive Cancer Center) |
|
Phase 1 Clinical |
Uclas Jonsson Comprehensive Cancer Center |
Candidiasis, Vulvovaginal; Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| LUCAR-G39P |
LUCAR-G39P |
Phase 1 Clinical |
|
Lymphoma, Non-Hodgkin |
Details
|
| Allogenic Chimeric Antigen Receptor(CAR)-T Cell Therapy(Beijing Cancer Hospital) |
LUCAR-20SP |
Phase 1 Clinical |
Beijing Cancer Hospital |
Lymphoma, Non-Hodgkin |
Details
|
| Rituximab biosimilar (Mabscale) |
|
Phase 1 Clinical |
Mabscale LLC |
Arthritis, Rheumatoid |
Details
|
| ASP-2802 |
ASP2802; ASP-2802 |
Phase 1 Clinical |
Astellas Pharma Global Development Inc |
Lymphoma, B-Cell |
Details
|
| JNJ-1493 |
JNJ-1493 |
Phase 1 Clinical |
Xencor Inc |
Hematologic Neoplasms; Lymphoma, B-Cell |
Details
|
| C-CAR168 CAR T-cell therapy (AbelZeta) |
C-CAR168 |
Phase 1 Clinical |
AbelZeta Pharma Inc |
Multiple Sclerosis, Relapsing-Remitting; Autoimmune Diseases; Neuromyelitis Optica; Lupus Erythematosus, Systemic; Muscular Diseases |
Details
|
| SCTB-35 |
SCTB35; SCTB-35 |
Phase 1 Clinical |
SinoCelltech Ltd |
Lymphoma, B-Cell |
Details
|
| Recombinant human anti-CD20 momoclonal antibody(Shanghai Crosslink Pharma) |
|
Phase 1 Clinical |
Shanghai Crosslink Pharmaceutical R & D Co Ltd |
Myasthenia Gravis; Pemphigus |
Details
|
| Anti-CD19 and Anti-CD20 Bicistronic Chimeric Antigen Receptor T Cells(NIH) |
|
Phase 1 Clinical |
National Cancer Institute |
Lymphoma, B-Cell; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| RO-7121932 |
RO7121932; RO-7121932; RO 7121932; RG-6035 |
Phase 1 Clinical |
Genentech Inc |
Multiple Sclerosis |
Details
|
| TanCART19/20 |
|
Phase 1 Clinical |
Pla General Hospital |
Neuromyelitis Optica |
Details
|
| LCAR-AIO |
LCAR-AIO; VHH CAR-T |
Phase 1 Clinical |
|
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma |
Details
|
| CAR-20-19-22 |
CAR-20-19-22 |
Phase 1 Clinical |
The Medical College Of Wisconsin Nonprofit |
Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Rituximab biosimilar (Zhejiang Teruisi Pharmaceutical) |
TRS001 |
Phase 1 Clinical |
Zhejiang Teruisi Pharmaceutical Inc |
Lymphoma |
Details
|
| QLP-31907 |
QLP-31907; QLP31907; PSB-202 |
Phase 1 Clinical |
Qilu Pharmaceutical Co Ltd |
Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Waldenstrom Macroglobulinemia; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| ADI-001 |
ADI-001 |
Phase 1 Clinical |
|
Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Primary mediastinal B cell lymphoma; Lymphoma, Non-Hodgkin; Burkitt Lymphoma |
Details
|
| TQB-2825 |
TQB-2825 |
Phase 1 Clinical |
Wuxi Biologics Co Ltd |
Hematologic Neoplasms |
Details
|
| CD19/CD20 CAR-T Cell Therapy (PersonGen) |
|
Phase 1 Clinical |
Persongen Biotherapeutics |
|
Details
|
| JMT-601 |
JMT-601 |
Phase 1 Clinical |
Shanghai Jmt-Bio Inc |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin |
Details
|
| LY007 |
LY007 |
Phase 1 Clinical |
Shanghai Longyao Biological Technology Co Ltd |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin |
Details
|
| Autologous humanized anti-CD19 and anti-CD20 dual specific CAR-T cell therapy (First Affiliated Hospital of Zhejiang University) |
|
Phase 1 Clinical |
First Affiliated Hospital Of Zhejiang University |
Lymphoma, Large B-Cell, Diffuse |
Details
|
| Plamotamab |
XmAb-13676 |
Phase 1 Clinical |
Xencor Inc |
Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| CD20/CD22 dual Targeted CAR T-cell therapy (Zhejiang University) |
|
Phase 1 Clinical |
Zhejiang University, Shanghai YaKe Biotechnology Co Ltd |
Hematologic Neoplasms |
Details
|
| Rituximab/Paclitaxel |
AR-160 |
Phase 1 Clinical |
Mayo Clinic |
Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Anti-CD20 CAR-T cell therapy (Wuhan Bio-Raid) |
|
Clinical |
Wuhan BioRaid Biotechnology Co Ltd |
Neoplasms |
Details
|
| Rituximab biosimilar(Bioxpress) |
BXT-2336 |
Clinical |
Bioxpress Therapeutics Sa |
Granulomatosis with Polyangiitis; Microscopic Polyangiitis; Arthritis, Rheumatoid; Lymphoma, Non-Hodgkin; Pemphigus; Leukemia, Lymphocytic, Chronic, B-Cell |
Details
|
| Rituximab/Bendamustine/Cytarabine |
|
|
Fondazione Italiana Linfomi Onlus |
|
Details
|
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one. Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service? Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!