





























 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!

This protein carries a polyhistidine tag at the C-terminus. The protein has a calculated MW of 55.6 kDa. The protein migrates as 60-70 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation. This protein has been verified not recognized by Trastuzumab in functional ELISA at ACRObiosystems.
>95% as determined by SDS-PAGE.
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
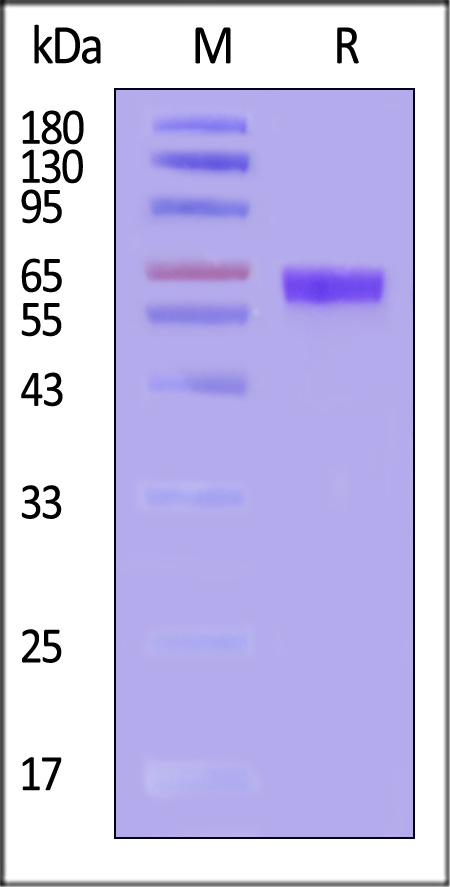
Human Her2 (23-510), His Tag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).

The purity of Human Her2 (23-510), His Tag (Cat. No. HE2-H52H9) is more than 85% and the molecular weight of this protein is around 55-70 kDa verified by SEC-MALS.

Immobilized Human Her2 (23-510), His Tag (Cat. No. HE2-H52H9) at 1 μg/mL (100 μL/well) can bind Pertuzumab Biosimilar with a linear range of 0.1-4 ng/mL (QC tested).

Immobilized Human Her2 (23-510), His Tag (Cat. No. HE2-H52H9) at 1 μg/mL (100 μL/well) can bind Bispecific Antibody (Her2 × Her3) with a linear range of 0.2-3 ng/mL (Routinely tested).

Immobilized Human Her2 (23-510), His Tag (Cat. No. HE2-H52H9) at 1 μg/mL (100 μL/well) can bind Pertuzumab with a linear range of 0.2-3 ng/mL,This protein has been verified not recognized by Trastuzumab in functional ELISA at ACRObiosystems. (Routinely tested).

ACROBiosystemsは、二重特異性抗体の治療薬への臨床開発を加速するために、高い生物活性を持つ均一なCD3δ/CD3εおよびCD3γ/CD3εタンパク質のシリーズを開発しました。

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox は、即使用可能のオルガノイド、オルガノイド分化ツールキット、創薬プロジェクトの進行を加速する、さまざまなサービスを含むオルガノイドソリューションのことです。

IL-15、IL-7、IL-21など、高品質のGMP準拠サイトカイン製品を提供しており、免疫細胞治療薬の臨床研究をサポートし、医薬品規制当局の承認を加速できます。

注目されている50以上のCAR-Tターゲットがあり、CARの発現の検出のために設計された抗原タンパク質をフローサイトメトリーで検証し、CARの発現が高い特異性で検出できます。ロット間の一貫性が保証されています。また、PE / FITC標識タンパク質を利用することで、ワンステップ染色でCARの発現を高い特異性でバックグラウンドなしに検出できます。

CD20、Claudin18.2、CD133、GPRC5D、CCR5、CCR8などの安定で高活性な全長型膜貫通タンパク質は、免疫学、ELISA、SPR、BLI、細胞実験、CAR陽性率検出に利用できます。VLP、界面活性剤、ナノディスクなどのテクニカルプラットフォームによって複数回膜貫通タンパク質の医薬品創薬を促進できます。

GMP準拠サイトカイン、高品質の細胞活性化/増幅試薬、遺伝子改変試薬/酵素、CAR検出試薬などの製品を提供し、細胞、遺伝子治療用に全体的なソリューションを提供し、創薬から臨床研究までのすべての段階をサポートします。

これまでに知られている免疫チェックポイント分子をほぼ製品化しており、様々なタグの製品群を提供できます。天然高分子の構造はMALSによって、生物活性はELISA/SPR/BLI/FACSなどによって検証済みです。またBiotin/FITCなどの標識も選択でき、抗体のハイスループットスクリーニングに利用できます。

弊社ACROBiosystemsはADC医薬品の開発のサポートに取り組み、注目されている様々なターゲットのために、異なる種類とタグの製品が開発され、高い純度と親和性が特長です。免疫、抗体スクリーン、SPR、細胞活性検査などの実験に利用できます。Protocolも無償で提供いたします。

すべての分子のFc受容体タンパク質だけでなく、一般的な変異体やビオチン標識タイプも含まれております。お客様のモノクローナル抗体の開発をサポートします。

インターロイキン、成長因子、ケモカイン、TNF など、様々なサイトカインターゲットの自然なコンフォメーションを確保するために HEK293 によって発現されます。SDS-PAGE/HPLC/SEC-MALS によって高純度は確認され、高い生物活性は ELISA/SPR/BLI によって確認されています。

AneuroはACROBiosystemsが神経科学研究のために設計した製品群のブランドです。神経科学研究を推進するための治療・診断用研究タンパク質、PFF、組み換え神経因子など、高品質の重要なタンパク質を提供しております。
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Osimertinib Mesylate | AZD-9291; RDL94R2A16; AZD-9291 Mesylate; AZD9291 | Approved | Astrazeneca Plc | 泰瑞沙, Tagrisso | United States | Carcinoma, Non-Small-Cell Lung | Astrazeneca Pharmaceutical Co Ltd | 2015-11-13 | Uterine Neoplasms; Urinary Bladder Neoplasms; Multiple Myeloma; Prostatic Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Meningeal Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Brain metastases; Adenocarcinoma of Lung; Endometrial Neoplasms; Thyroid Neoplasms; Carcinoma, Squamous Cell; Lymphoma; Glioma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Adenocarcinoma; Melanoma; Esophageal Neoplasms; Liver Neoplasms; Head and Neck Neoplasms; Neoplasm, Residual; Ovarian Neoplasms; Hematologic Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Solid tumours; Neoplasms; Glioblastoma; Colonic Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Skin Neoplasms; Small Cell Lung Carcinoma; Pancreatic Neoplasms | Details |
| Trastuzumab biosimilar (Celltrion) | CT-P6; CT-P06 | Approved | Celltrion Inc | Herzuma | South Korea | Stomach Neoplasms; Breast Neoplasms | null | 2014-01-01 | Stomach Neoplasms; Breast Neoplasms | Details |
| Trastuzumab emtansine biosimilar (Zydus Cadila) | ZRC-3256 | Approved | Zydus Cadila | Ujvira | India | Breast Neoplasms | Zydus Cadila | 2021-05-24 | Breast Neoplasms | Details |
| Trastuzumab biosimilar (Zhejiang Hisun Pharmaceutical) | HS-022 | Approved | Zhejiang Hisun Pharmaceutical Co Ltd | 安瑞泽 | Mainland China | Stomach Neoplasms; Breast Neoplasms | Hisun Biopharmaceutical Co Ltd | 2023-02-28 | Stomach Neoplasms; Breast Neoplasms | Details |
| Trastuzumab biosimilar (Samsung Bioepis) | AMT-901; SB-3 | Approved | Samsung Bioepis Co Ltd | Samfenet, Ontruzant | EU | Stomach Neoplasms; Breast Neoplasms | Samsung Bioepis Nl Bv | 2017-11-15 | Stomach Neoplasms; Breast Neoplasms | Details |
| Afatinib Dimaleate | BIBW-2992-MA2; BIBW-2992 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Gilotrif, Giotrif, Tovok, 吉泰瑞, Tomtovok | United States | Carcinoma, Non-Small-Cell Lung | C.H. Boehringer Sohn Ag & Co. Kg | 2013-07-12 | Esophageal Squamous Cell Carcinoma; Urethral Neoplasms; Brain Neoplasms; Prostatic Neoplasms; Ureteral Neoplasms; Urologic Neoplasms; Colorectal Neoplasms; Lung Neoplasms; Carcinoma, Squamous Cell; Breast Neoplasms; Gallbladder Neoplasms; Uterine Neoplasms; Lymphoma; Glioma; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Neoplasm Metastasis; Small Cell Lung Carcinoma; Head and Neck Neoplasms; Solid tumours; Hematologic Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Stomach Neoplasms; Renal Insufficiency; Neoplasms; Rhabdomyosarcoma; Neuroectodermal Tumors; Glioblastoma; Neoplasms, Squamous Cell; Urinary Bladder Neoplasms; Chordoma; Liver Diseases; Multiple Myeloma | Details |
| Trastuzumab biosimilar (Synthon) | ABP-980 | Approved | Synthon Bv | Kanjinti | EU | Breast Neoplasms; Stomach Neoplasms | Amgen Europe Bv | 2018-05-16 | Urinary Bladder Neoplasms; Melanoma; Adenocarcinoma; Uterine Cervical Neoplasms; Esophageal adenocarcinoma; Thyroid Neoplasms; Uterine Neoplasms; Endometrial Neoplasms; Lung Neoplasms; Lymphoma; Glioma; Colorectal Neoplasms; Carcinoma, Intraductal, Noninfiltrating; Cholangiocarcinoma; Prostatic Neoplasms; Breast Neoplasms; Liver Neoplasms; Multiple Myeloma; Colonic Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Skin Neoplasms; Carcinoma, Renal Cell; Rectal Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Esophageal Neoplasms; Hematologic Neoplasms; Head and Neck Neoplasms; Ovarian Neoplasms; Solid tumours | Details |
| Trastuzumab biosimilar (Zydus) | Approved | Zydus Cadila | Vivitra | India | Breast Neoplasms | Zydus Cadila | 2016-01-01 | Breast Neoplasms | Details | |
| Margetuximab | MGAH-22 | Approved | Macrogenics Inc | Margenza | United States | Breast Neoplasms | Macrogenics Inc | 2020-12-16 | Ovarian Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Digestive System Neoplasms; Urinary Bladder Neoplasms; Breast Neoplasms; Metastatic breast cancer; Neoplasm Metastasis; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Trastuzumab/Pertuzumab | RO-7198574; RG-6264 | Approved | F. Hoffmann-La Roche Ltd | Phesgo, 赫捷康 | United States | Breast Neoplasms | Genentech Inc | 2020-06-29 | Breast Neoplasms; Colorectal Neoplasms; Metastatic breast cancer | Details |
| Trastuzumab biosimilar (Reliance Life Sciences) | R-TPR-016 | Approved | Reliance Life Sciences | TrastuRel | India | Breast Neoplasms | Reliance Life Sciences | 2015-01-01 | Breast Neoplasms | Details |
| Trastuzumab biosimilar (Anke Biotech) | Approved | Anhui Anke Biotechnology (Group) Co Ltd | Mainland China | Breast Neoplasms | Anhui Anke Biotechnology (Group) Co Ltd | 2023-10-27 | Breast Neoplasms | Details | ||
| Inetetamab | CMAB-302; 302H | Approved | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Cipterbin, 赛普汀 | Mainland China | Breast Neoplasms | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | 2020-06-17 | Breast Neoplasms | Details |
| Trastuzumab biosimilar (Pfizer) | PF-5280014; PF-05280014 | Approved | Pfizer Inc | Trazimera, trastuzumab-qyyp | EU | Breast Neoplasms; Stomach Neoplasms | Pfizer Europe Ma Eeig | 2018-07-26 | Colonic Neoplasms; Melanoma; Uterine Cervical Neoplasms; Lung Neoplasms; Lymphoma; Thyroid Neoplasms; Uterine Neoplasms; Endometrial Neoplasms; Glioma; Colorectal Neoplasms; Prostatic Neoplasms; Breast Neoplasms; Cholangiocarcinoma; Multiple Myeloma; Urinary Bladder Neoplasms; Head and Neck Neoplasms; Carcinoma, Ovarian Epithelial; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Skin Neoplasms; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Esophageal Neoplasms; Solid tumours; Hematologic Neoplasms; Liver Neoplasms; Ovarian Neoplasms | Details |
| Trastuzumab biosimilar (CTTQ Pharma) | TQ-B211 | Approved | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 赛妥 | Mainland China | Breast Neoplasms; Stomach Neoplasms | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | 2023-07-25 | Stomach Neoplasms; Breast Neoplasms; Metastatic breast cancer | Details |
| Trastuzumab biosimilar (AXXO) | Approved | Axxo | Breast Neoplasms | Details | ||||||
| Trastuzumab biosimilar (Biocon/Mylan) | Bmab-200; Myl-1401O; HerMyl-1401O | Approved | Biocon Ltd, Mylan Nv | CANMAb, Ogivri, Zedora, Hertraz | India | Breast Neoplasms; Esophageal Neoplasms; Stomach Neoplasms | null | 2013-01-01 | Esophageal Neoplasms; Stomach Neoplasms; Breast Neoplasms; Carcinoma, Intraductal, Noninfiltrating; Esophageal adenocarcinoma; Uterine Cervical Neoplasms; Adenocarcinoma | Details |
| Trastuzumab | R-597; RO45-2317; Anti-HER2/neu-MAb; huMAb4D5-8; RG-597; MKC-454 | Approved | Genentech Inc | Herceptin, 赫赛汀 | United States | Breast Neoplasms | Genentech Inc | 1998-09-25 | Breast Neoplasms; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis; Adenocarcinoma; Vulvar Diseases; Lung Neoplasms; Endometrial Neoplasms; Metastatic breast cancer; Urologic Neoplasms; Carcinoma, Mucoepidermoid; Carcinoma, Acinar Cell; Sarcoma; Urethral Neoplasms; Prostatic Neoplasms; Neoplastic Cells, Circulating; Urinary Bladder Neoplasms; Carcinoma, Transitional Cell; Salivary Gland Neoplasms; Colonic Neoplasms; Neoplasms; Small Cell Lung Carcinoma; Meningeal Carcinomatosis; Esophageal Neoplasms; Stomach Neoplasms; Solid tumours; Ependymoma; Head and Neck Neoplasms | Details |
| Trastuzumab biosimilar (Intas Pharmaceuticals) | Approved | Intas Biopharmaceuticals | Breast Neoplasms | Details | ||||||
| Tucatinib | ARRY-380; ONT-380; MK-7119 | Approved | Array Biopharma | Tukysa | United States | Breast Neoplasms | Seagen Inc | 2020-04-17 | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Meningeal Neoplasms; Colorectal Neoplasms; Hepatic Insufficiency; Metastatic breast cancer; Neoplasm Metastasis | Details |
| Ado-trastuzumab emtansine | RG-3502; R-3502; T-DM1; PRO-132365; RO-5304020 | Approved | Genentech Inc | Kadcyla, 赫赛莱 | United States | Breast Neoplasms | Genentech Inc | 2013-02-22 | Solid tumours; Hematologic Neoplasms; Stomach Neoplasms; Pancreatic Neoplasms; Salivary Gland Neoplasms; Small Cell Lung Carcinoma; Urinary Bladder Neoplasms; Multiple Myeloma; Breast Neoplasms; Cholangiocarcinoma; Urologic Neoplasms; Lung Neoplasms; Lymphoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Trastuzumab biosimilar (Dr. Reddy's Laboratories) | Approved | Dr.Reddy's Laboratories Ltd | Hervycta | India | Breast Neoplasms | Dr.Reddy's Laboratories Ltd | 2018-07-26 | Breast Neoplasms | Details | |
| Pertuzumab | rhuMab-2C4; RO-4368451; R-1273; RG-1273; RO-4368451-F01; rhuMAb2C4; Ro 436-8451/F01; RO4368451 | Approved | Genentech Inc, F. Hoffmann-La Roche Ltd | 帕捷特, Perjeta, Omnitarg | United States | Breast Neoplasms | Genentech Inc | 2012-06-08 | Neuroendocrine Tumors; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Multiple Endocrine Neoplasia; Fallopian Tube Neoplasms; Carcinoma, Neuroendocrine; Peritoneal Neoplasms; Prostatic Neoplasms; Neuroblastoma; Breast Neoplasms; Adrenal Gland Neoplasms; Ovarian Neoplasms; Neoplasms; Pancreatic neuroendocrine tumors (pNET); Pancreatic Neoplasms; Colonic Neoplasms; Carcinoid Tumor; Stomach Neoplasms; Esophageal Neoplasms; Rectal Neoplasms; Solid tumours | Details |
| Trastuzumab/Hyaluronidase | Approved | Genentech Inc | Herceptin Hylecta | United States | Breast Neoplasms | Genentech Inc | 2019-02-28 | Breast Neoplasms | Details | |
| Fam-trastuzumab deruxtecan | DS-8201a; DS-8201; VRN-101099 | Approved | Daiichi Sankyo Co Ltd | Enhertu, 优赫得, 優赫得 | United States | Breast Neoplasms | Daiichi Sankyo Co Ltd | 2019-12-20 | Osteosarcoma; Neoplasm Metastasis; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Endometrial Neoplasms; Brain metastases; Metastatic breast cancer; Colorectal Neoplasms; Meningeal Neoplasms; Solid tumours; Brain Neoplasms; Breast Neoplasms; Carcinoma, Transitional Cell; Triple Negative Breast Neoplasms; Glioblastoma; Neoplasms; Neoplasms, Glandular and Epithelial; Stomach Neoplasms; Esophageal Neoplasms | Details |
| Trastuzumab biosimilar (Shanghai Henlius Biotech) | HLX-02 | Approved | Shanghai Henlius Biotech Inc | Zercepac, 汉曲优 | EU | Breast Neoplasms; Stomach Neoplasms | Accord Healthcare Slu | 2020-07-27 | Stomach Neoplasms; Neoplasms; Breast Neoplasms | Details |
| Sunvozertinib | DZD-9008 | Approved | Dizal (Jiangsu) Pharmaceutical Co Ltd | 舒沃哲 | Mainland China | Carcinoma, Non-Small-Cell Lung | Dizal (Jiangsu) Pharmaceutical Co Ltd | 2023-08-22 | Lymphoma, B-Cell; Hepatic Insufficiency; Lymphoma, Non-Hodgkin; Carcinoma, Non-Small-Cell Lung | Details |
| Trastuzumab biosimilar (Biocad) | BCD-022 | Approved | Biocad | HERtiCAD | Russian Federation | Breast Neoplasms | Biocad | 2016-01-01 | Breast Neoplasms | Details |
| Neratinib Maleate | CAN-030; PB-272; HKI-272; PF-0528767; WAY-179272 | Approved | Pfizer Inc | Nerlynx, 贺俪安 | United States | Breast Neoplasms | Puma Biotechnology Inc | 2017-07-17 | Solid tumours; Esophageal Neoplasms; Neoplasms; Breast Neoplasms; Hepatic Insufficiency; Colorectal Neoplasms; Lung Neoplasms; Brain metastases; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| Lapatinib Ditosylate Hydrate | GW-572016; GW-572016F; GW-2016 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | Tykerb, Tyverb, 泰立沙, Tykerb/Tyverb | United States | Breast Neoplasms | Novartis Pharma Ag | 2007-03-13 | Laryngeal Neoplasms; Prostatic Neoplasms; Gliosarcoma; Colorectal Neoplasms; Astrocytoma; Bile Duct Neoplasms; Carcinoma, Acinar Cell; Carcinoma, Mucoepidermoid; Endometrial Neoplasms; Neoplasms, Gonadal Tissue; Lung Neoplasms; Carcinoma, Squamous Cell; Glioma; Brain Neoplasms; Gallbladder Neoplasms; Brain metastases; Lymphoma; Uterine Cervical Neoplasms; Adenocarcinoma; Melanoma; Tongue Neoplasms; Breast Neoplasms, Male; Carcinoma, Non-Small-Cell Lung; Neuroma, Acoustic; Carcinoma, Hepatocellular; Neoplasm Metastasis; Glioblastoma; Ovarian Neoplasms; Ependymoma; Head and Neck Neoplasms; Liver Neoplasms; Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Carcinoma; Abdominal Neoplasms; Salivary Gland Neoplasms; Medulloblastoma; Carcinoma, Verrucous; Neoplasms; Neurofibromatosis 2; Small Cell Lung Carcinoma; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Central Nervous System Neoplasms; Urinary Bladder Neoplasms; Oligodendroglioma; Ca | Details |
| Trastuzumab biosimilar (EirGenix/Sandoz) | EG-12014; EGI 014A1; EGI-014 | Approved | Sandoz, Eirgenix Inc | EU | Breast Neoplasms; Stomach Neoplasms | Sandoz Gmbh | 2023-11-15 | Stomach Neoplasms; Breast Neoplasms | Details | |
| Trastuzumab biosimilar (AryoGen Pharmed) | Approved | Aryogen Biopharma | AryoTrust | Stomach Neoplasms; Breast Neoplasms | Details | |||||
| Disitamab Vedotin | RC-48-ADC; RC48-ADC; RC-48 | Approved | RemeGen Co Ltd | 爱地希, Aidixi | Mainland China | Stomach Neoplasms | RemeGen Co Ltd | 2021-06-08 | Prostatic Neoplasms, Castration-Resistant; Melanoma; Uterine Cervical Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Metastatic breast cancer; Endometrial Neoplasms; Genital Neoplasms, Female; Breast Diseases; Bile Duct Neoplasms; Breast Neoplasms; Liver Neoplasms; Urinary Bladder Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Genital Diseases, Female; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Esophageal Neoplasms; Ovarian Neoplasms; Biliary Tract Neoplasms; Solid tumours | Details |
| Pyrotinib Maleate | HTI-1001; SHR-1258; BLTN | Approved | Jiangsu Hengrui Medicine Co Ltd | 艾瑞妮 | Mainland China | Breast Neoplasms | Jiangsu Hengrui Medicine Co Ltd | 2018-08-12 | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Adenocarcinoma of Lung; Neoplasms; Breast Neoplasms; Bile Duct Neoplasms; Lung Neoplasms; Metastatic breast cancer; Gastrointestinal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Dacomitinib | PF-00299804-03; PF-00299804-3; PF-00299804; PF-299804; PF-299; PF-804 | Approved | Pfizer Inc | Vizimpro | United States | Carcinoma, Non-Small-Cell Lung | Pfizer Inc | 2018-09-27 | Brain Neoplasms; Adenocarcinoma; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Penile Neoplasms; Carcinoma, Squamous Cell; Esophageal Squamous Cell Carcinoma; Mouth Neoplasms; Lung Neoplasms; Colorectal Neoplasms; Solid tumours; Liver Diseases; Carcinoma, Large Cell; Glioblastoma; Neoplasms; Small Cell Lung Carcinoma; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Head and Neck Neoplasms | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| PLB-1004 | PLB-1004 | Phase 3 Clinical | Beijing Avistone Pharmaceuticals Biotechnology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| Mefatinib | Phase 3 Clinical | Suzhou Maitai Bio-Technology Co Ltd | Solid tumours; Carcinoma, Non-Small-Cell Lung | Details | |
| Recombinant anti-HER2 humanized monoclonal antibody (Shanghai Institute of Biological Products) | SIBP-01 | Phase 3 Clinical | Shanghai Institute Of Biological Products Co Ltd | Stomach Neoplasms; Breast Neoplasms | Details |
| Anvatabart opadotin | ARX-788; NCB-001 | Phase 3 Clinical | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Triple Negative Breast Neoplasms; Breast Neoplasms; Bile Duct Neoplasms; Metastatic breast cancer | Details | |
| Hemay-022 | Hemay-022 | Phase 3 Clinical | Tianjin Hemay Pharmaceutical Co Ltd, Xiajiang Hemei Pharmaceutical Co Ltd | Breast Neoplasms; Foodborne Diseases | Details |
| Trastuzumab biosimilar (Hanwha Biologics) | HD-201 | Phase 3 Clinical | Hanwha Biologics | Breast Neoplasms | Details |
| Pertuzumab biosimilar(Hisun Pharm) | HS-627; HS627 | Phase 3 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd, Beijing Mabworks Biotech Co Ltd | Breast Neoplasms | Details |
| Trastuzumab rezetecan | SHR-A1811 | Phase 3 Clinical | Jiangsu Hengrui Medicine Co Ltd, Suzhou Suncadia Biopharmaceuticals Co Ltd, Shanghai Hengrui Pharmaceutical Co Ltd | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Triple Negative Breast Neoplasms; Neoplasms; Salivary Gland Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Genital Neoplasms, Female; Metastatic breast cancer; HR-positive breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| Trastuzumab monomethyl auristatin F | FS-1502; LCB14-0110; LCB-14-0110 | Phase 3 Clinical | Legochembio | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| Varlitinib Ditosylate | SPS-4370; ASLAN-001; ARRY-334543; ARRY-543; QBT-01 | Phase 3 Clinical | Array Biopharma | Biliary Tract Neoplasms; Solid tumours; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms; Breast Neoplasms; Cholangiocarcinoma; Bile Duct Neoplasms | Details |
| Anbenitamab | KN-026 | Phase 3 Clinical | Suzhou Alphamab Co Ltd | Biliary Tract Neoplasms; Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Metastatic breast cancer; Lymphoma; Gastrointestinal Neoplasms | Details |
| Coprelotamab | GB-221 | Phase 3 Clinical | Genor Biopharma Co Ltd | Breast Neoplasms | Details |
| Trastuzumab vedotin | MRG-002 | Phase 3 Clinical | Solid tumours; Biliary Tract Neoplasms; Stomach Neoplasms; Esophageal Neoplasms; Carcinoma, Transitional Cell; Breast Neoplasms; Bile Duct Neoplasms; Metastatic breast cancer; Adenocarcinoma; Carcinoma, Non-Small-Cell Lung | Details | |
| trastuzumab biosimilar (Hetero) | Ado-Trastuzumab biosimilar (Hetero) | Phase 3 Clinical | Hetero Drugs Ltd | Neoplasms | Details |
| BL-M07D1 | BLM07D1 | Phase 3 Clinical | SystImmune | Solid tumours; Biliary Tract Neoplasms; Ovarian Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Breast Neoplasms; Genital Neoplasms, Female; Endometrial Neoplasms; Urogenital Neoplasms; Carcinoma, Non-Small-Cell Lung; Gastrointestinal Neoplasms; Uterine Cervical Neoplasms | Details |
| Pertuzumab biosimilar(Zydus Cadila) | ZRC-3277 | Phase 3 Clinical | Zydus Cadila | Breast Neoplasms | Details |
| HL-02 | HL-02 | Phase 3 Clinical | Hualan Genetic Engineering Co Ltd | Breast Neoplasms | Details |
| JSKN-003 | JSKN-003 | Phase 3 Clinical | Alphamab Oncology | Solid tumours; Neoplasms; Breast Neoplasms | Details |
| Pertuzumab biosimilar (Shanghai Henlius Biotech) | HLX-11 | Phase 3 Clinical | Shanghai Henlius Biotech Inc | Breast Neoplasms; Metastatic breast cancer | Details |
| HER2/Neu GP2 vaccine(Greenwich LifeSciences Inc/NuGenerex Immuno Oncology) | GLSI-100 | Phase 3 Clinical | Antigen Express Inc, Norwell Inc | Breast Neoplasms | Details |
| DB-1303 | BNT323; DB-1303 | Phase 3 Clinical | Solid tumours; Breast Neoplasms; Metastatic breast cancer; Endometrial Neoplasms; Neoplasm Metastasis | Details | |
| Pertuzumab biosimilar (Jushi Biopharmaceutical ) | SYSA1901; SYSA-1901 | Phase 3 Clinical | Jushi Biopharmaceutical Co Ltd | Breast Neoplasms; Metastatic breast cancer | Details |
| Trastuzumab biosimilar(Aprogen/Nichi-Iko) | AP-06 | Phase 3 Clinical | Nichi-Iko Pharmaceutical Co Ltd, Aprogen | Breast Neoplasms | Details |
| Zongertinib | BI-1810631 | Phase 3 Clinical | Boehringer Ingelheim Gmbh | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Breast Neoplasms; Esophageal adenocarcinoma; Lung Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma | Details |
| Pertuzumab biosimilar (Xuanzhu/SL) | KM-118; KM118; XZP-KM118 | Phase 3 Clinical | Xuanzhu (Shijiazhuang) Biotechnology Co Ltd, Beijing Sl Pharmaceutical Co Ltd | Breast Neoplasms | Details |
| Pertuzumab biosimilar(Biocon) | Phase 3 Clinical | Biocon Ltd | Breast Neoplasms | Details | |
| Pertuzumab biosimilar (CinnaGen) | Phase 3 Clinical | Cinnagen | Breast Neoplasms | Details | |
| DP-303c | DP-303c; DP303c | Phase 3 Clinical | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Salivary Gland Neoplasms; Neoplasms; Breast Neoplasms; Metastatic breast cancer | Details | |
| Larotinib Mesylate | Z-650 | Phase 3 Clinical | Guangdong Dongyangguang Pharmaceutical Co Ltd | Solid tumours; Esophageal Neoplasms; Pancreatic Neoplasms; Esophageal Squamous Cell Carcinoma | Details |
| DF-1001 | DF-1001 | Phase 2 Clinical | Dragonfly Therapeutics Llc | Solid tumours | Details |
| TPIV-100 (TapImmune) | TPIV-100 | Phase 2 Clinical | Mayo Clinic | Breast Neoplasms; Carcinoma, Intraductal, Noninfiltrating; Breast Neoplasms, Male | Details |
| BB-1701 | BB-1701 | Phase 2 Clinical | Bliss Biopharmaceutical (Hangzhou) Co Ltd | Solid tumours; Stomach Neoplasms; Carcinoma, Transitional Cell; Breast Neoplasms; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| Sapitinib | AZD-8931 | Phase 2 Clinical | Astrazeneca Plc | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung; Neoplasm Metastasis | Details |
| Anti-CD3 and anti-HER2 BiTE-expressing T cell (MedImmune) | Phase 2 Clinical | University Of Michigan, Huazhong University Of Science And Technology, University Of Virginia Cancer Center, Medimmune | Breast Neoplasms | Details | |
| AU-101 | AU-101 | Phase 2 Clinical | Aurora Biopharma | Glioblastoma; Osteosarcoma; Breast Neoplasms | Details |
| Tesevatinib | XL-647; KD-020; KD-019; EXEL-7647 | Phase 2 Clinical | Exelixis Inc | Glioblastoma; Neoplasms; Brain Neoplasms; Breast Neoplasms; Polycystic Kidney, Autosomal Dominant; Brain metastases; Polycystic Kidney, Autosomal Recessive; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Recombinant anti-HER2 antibody-Tub114 | DAC-001; DX126-262 | Phase 2 Clinical | Hangzhou Dac Biotech Company Ltd | Stomach Neoplasms; Carcinoma, Transitional Cell; Breast Neoplasms; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| AMX-3009 Maleate | AMX-3009; AMX3009马来酸 | Phase 2 Clinical | Anrun Medicine Technology (Suzhou) Co Ltd | Solid tumours; Squamous Cell Carcinoma of Head and Neck; Stomach Neoplasms; Pancreatic Neoplasms; Breast Neoplasms; Neoplasm Metastasis; Carcinoma, Non-Small-Cell Lung | Details |
| Allitinib Tosylate | AST-6; ALS-1306; AST-1306 | Phase 2 Clinical | Shanghai Allist Pharmaceutical Technology Co Ltd | Breast Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| HLX-22 | AC-101; HLX-22 | Phase 2 Clinical | Shanghai Henlius Biotech Inc, Abclon Inc | Solid tumours; Stomach Neoplasms; Breast Neoplasms | Details |
| Cinrebafusp alfa | PRS-343 | Phase 2 Clinical | Pieris Pharmaceuticals | Solid tumours; Stomach Neoplasms; Urinary Bladder Neoplasms; Breast Neoplasms | Details |
| AU-105 | AU-105 | Phase 2 Clinical | Aurora Biopharma | Glioblastoma | Details |
| Selatinib Ditosilate | QLNC-120 | Phase 2 Clinical | Qilu Antibiotics (Linyi) Pharmaceutical Co Ltd, Qilu Pharmaceutical Co Ltd | Breast Neoplasms | Details |
| HER2 cancer vaccine (BioLife Science) | PEV-6; PEV-6A; IMU-131 | Phase 2 Clinical | Pevion Biotech Ltd | Esophageal Neoplasms; Stomach Neoplasms; Adenocarcinoma; Gastrointestinal Neoplasms | Details |
| Trastuzumab imbotolimod | BDC-1001 | Phase 2 Clinical | Bolt Biotherapeutics Inc | Solid tumours; Esophageal Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Endometrial Neoplasms | Details |
| Zenocutuzumab | MCLA-128 | Phase 2 Clinical | Merus Nv | Solid tumours; Pancreatic Neoplasms; Prostatic Neoplasms, Castration-Resistant; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| AE-37/GP-2 vaccine | AE-37 (Generex Biotechnology Corporation) | Phase 2 Clinical | Generex Biotechnology Corp | Triple Negative Breast Neoplasms; Breast Neoplasms | Details |
| ORIC-114 | ORIC-114; VRN-07 | Phase 2 Clinical | Voronoi Inc | Solid tumours; Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| YH-32367 | ABL105; YH-32367 | Phase 2 Clinical | Abl Bio Inc | Solid tumours; Stomach Neoplasms; Breast Neoplasms | Details |
| Multiple 4SCAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Breast Neoplasms | Details | |
| HER2/neu peptide vaccine (Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium) | Phase 2 Clinical | Fred Hutchinson/University Of Washington Cancer Consortium | Breast Neoplasms; Breast Neoplasms, Male | Details | |
| Neratinib (Fukang (Shanghai) Health Technology) | CVL009; CVL-009 | Phase 2 Clinical | Solid tumours; Uterine Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| JSKN-033 | JSKN033; JSKN-033; JSKN-003/Envafolimab | Phase 2 Clinical | Jiangsu Alphamab Biopharmaceuticals Co Ltd | Solid tumours | Details |
| HER2 Targeted HypoSti.CAR-T Cells(Chinese PLA General Hospital) | Phase 2 Clinical | Pla General Hospital, Fudan University | Solid tumours | Details | |
| IBI-354 | IBI-354 | Phase 2 Clinical | Innovent Biologics(Suzhou) Co Ltd | Solid tumours | Details |
| TQB-2102 | TQB-2102; TQB2102 | Phase 2 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Neoplasms; Breast Neoplasms; Metastatic breast cancer | Details |
| TQB-2930 | TQB-2930 | Phase 2 Clinical | Nanjing Shunxin Pharmaceuticals Co Ltd Of Chiatai Tianqing Pharmaceutical Group | Solid tumours; Neoplasms; Breast Neoplasms | Details |
| BAY-2927088 | BAY-2927088; BAY2927088 | Phase 2 Clinical | Bayer AG, Broad Institute, Harvard University | Carcinoma, Non-Small-Cell Lung | Details |
| ST-1703 | ST-1703 | Phase 2 Clinical | Beijing Scitech-Mq Pharmaceuticals Ltd | Solid tumours; Metastatic breast cancer | Details |
| Iodine-131-SGMIB anti-HER2 monoclonal antibody | [131I]-SGMIB Anti-HER2-VHH1; 131I-SGMIB-anti-HER2-VHH1; Iodine-131-SGMIB-anti-HER2-VHH1; CAM-H2 | Phase 2 Clinical | Camel-Ids Nv | Stomach Neoplasms; Esophageal Neoplasms; Breast Neoplasms | Details |
| EO-1001 | NT-113; APL-122 | Phase 2 Clinical | Edison Pharmaceuticals Inc | Neoplasms | Details |
| IMM-2902 | IMM-2902 | Phase 2 Clinical | ImmuneOnco Biopharmaceuticals (Shanghai) Co Ltd | Solid tumours; Stomach Neoplasms; Breast Neoplasms; Cholangiocarcinoma; Lung Neoplasms | Details |
| AB-201 (Artiva Biotherapeutics) | Phase 2 Clinical | GC Cell Corp | Solid tumours; Esophageal Neoplasms; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Adenocarcinoma | Details | |
| Fencabtagene autoleucel | TAC01-HER2 | Phase 2 Clinical | Triumvira Immunologics Inc | Esophageal Neoplasms; Stomach Neoplasms | Details |
| Recombinant anti-HER2 humanized HuA21 monoclonal antibody(Hankemab) | HuA21; HuA-21 | Phase 2 Clinical | Hefei Hankemab Biotechnology Co Ltd | Solid tumours; Stomach Neoplasms | Details |
| JRF-103 | JRF103; JRF-103 | Phase 2 Clinical | Shenzhen Jinrui Foundation Biotechnology Co Ltd | Solid tumours | Details |
| IAH-0968 | IAH-0968; IAH0968 | Phase 2 Clinical | SunHo (China) BioPharmaceutical Co Ltd | Solid tumours; Biliary Tract Neoplasms; Stomach Neoplasms; Colorectal Neoplasms | Details |
| AIP-303 | AIP-303 | Phase 2 Clinical | Advanced Imaging Projects | Breast Neoplasms | Details |
| GQ-1001 | GQ-1001 | Phase 2 Clinical | GeneQuantum Healthcare (Suzhou) Co Ltd | Biliary Tract Neoplasms; Solid tumours; Breast Neoplasms; Metastatic breast cancer | Details |
| FCN-411 | FCN-411 | Phase 2 Clinical | Shanghai Fosun Pharmaceutical (Group) Co Ltd | Small Cell Lung Carcinoma; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Epertinib | S-222611 | Phase 2 Clinical | Shionogi & Co Ltd | Neoplasms | Details |
| Pirotinib Hydrochloride | KBP-5209 | Phase 2 Clinical | Shandong Xuanzhu Pharmaceutical Technology Co Ltd | Solid tumours; Breast Neoplasms; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Gancotamab | MM-302 | Phase 2 Clinical | Merrimack Pharmaceuticals Inc | Ovarian Neoplasms; Breast Neoplasms | Details |
| Anti-CD3-anti-HER2-activated T cells | Phase 2 Clinical | Transtarget | Prostatic Neoplasms, Castration-Resistant; Breast Neoplasms; Prostatic Neoplasms | Details | |
| HER2/neu cancer vaccine (University of Washington/Corixa/GSK) | Phase 2 Clinical | University Of Washington | Ovarian Neoplasms; Small Cell Lung Carcinoma; Breast Neoplasms; Lung Neoplasms; Breast Neoplasms, Male | Details | |
| anti-HER2 ADC (Pfizer) | PF-06804103 | Phase 2 Clinical | Pfizer Inc | Triple Negative Breast Neoplasms; Neoplasms; Breast Neoplasms | Details |
| ETBX-021 | ETBX-021 | Phase 2 Clinical | Nantbioscience Inc, Etubics Corp | Ovarian Neoplasms; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Prostatic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| HER2(EQ)BBzeta/CD19 T cells (City of Hope) | Phase 1 Clinical | Mustang Bio Inc, City Of Hope National Medical Center | Glioma | Details | |
| RG-6148 | RG-6148; DHES-0815A | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Breast Neoplasms | Details |
| CIDeCAR | Phase 1 Clinical | Bellicum Pharmaceuticals Inc, National Cancer Institute | Osteosarcoma | Details | |
| 99m-Tc labeled anti-HER2 single domain antibody (NanoMab) | 99mTc-NM-02 | Phase 1 Clinical | Nanomab Technology Ltd | Breast Neoplasms | Details |
| GB-235 | GB-235 | Phase 1 Clinical | Genor Biopharma Co Ltd | Breast Neoplasms | Details |
| HER-2/Neu pulsed DC1 vaccine (University of Pennsylvania) | Phase 1 Clinical | University Of Pennsylvania, National Cancer Institute | Breast Neoplasms | Details | |
| Anti-HER2 monoclonal antibody-MCC-DM1 conjugate (Shanghai Pharma) | B-003 (Shanghai Pharma) | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd, Shanghai Crosslink Pharmaceutical R & D Co Ltd | Breast Neoplasms | Details |
| MP-0274 | DARPin-41; SPA-28; CME-114; CME-115; CME-118; CME-119; MP-0274 | Phase 1 Clinical | Molecular Partners Ag | Neoplasms | Details |
| 68Ga-anti-HER2 nanobodies (Universite Libre de Bruxelles) | Phase 1 Clinical | Universite Libre De Bruxelles | Breast Neoplasms | Details | |
| KY-1701 | KY-1701 | Phase 1 Clinical | Jiangsu Kanion Pharmaceutical Co Ltd | Breast Neoplasms | Details |
| Recombinant anti-HER2 subdomain II humanized monoclonal antibody(Livzon) | LZM-005 | Phase 1 Clinical | Livzon Pharmaceutical Group Inc | Breast Neoplasms | Details |
| MX-402 | MX-402 | Phase 1 Clinical | Medvax Technologies | Stomach Neoplasms | Details |
| NJH-395 | NJH-395 | Phase 1 Clinical | Novartis Pharma Ag | Neoplasms | Details |
| MB-103 | MB-103 HER2 CAR; MB-103; HER2(EQ)BBζ/CD19t+ Tcm; HER2-BBζ CAR-T cells | Phase 1 Clinical | City Of Hope National Medical Center | Glioblastoma; Brain metastases | Details |
| Fidasimtamab | IBI-315 | Phase 1 Clinical | Hanmi Pharmaceutical Co Ltd, Innovent Biologics(Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| Trastuzumab biosimilar (NeuClone/Serum Institute of India) | Phase 1 Clinical | Serum Institute Of India Ltd, Neuclone | Stomach Neoplasms; Breast Neoplasms | Details | |
| RG-6596 | ZN-1041; RG-6596; ZN-A-1041 | Phase 1 Clinical | Suzhou Zanrong Pharmaceutical Technology Co Ltd | Solid tumours; Breast Neoplasms | Details |
| Recombinant anti-HER2 humanized monoclonal antibody complex | B-002 | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Breast Neoplasms | Details |
| Runimotamab | BTRC-4017A; RG-6194; RO-7227780 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms | Details |
| KSP-910638-G | KSP-910638G; KSP-910638-G | Phase 1 Clinical | University Of Michigan | Gastrointestinal Neoplasms | Details |
| TT-16 | TT-16 | Phase 1 Clinical | Tessa Therapeutics Ltd | Solid tumours | Details |
| SHR-A1201 | SHR-A1201 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Breast Neoplasms | Details |
| M-802(Wuhan Yzy Biopharma/CSPC Pharmaceutical) | M-802 | Phase 1 Clinical | Wuhan Yzy Biopharma Co Ltd, CSPC Pharmaceutical Group Ltd | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Breast Neoplasms | Details |
| BCD-147 | BCD-147 | Phase 1 Clinical | Biocad | Details | |
| Autologous-HER2-specific-T-cells | Autologous-HER2-specific-T-cells | Phase 1 Clinical | Baylor College Of Medicine | Sarcoma; Brain Neoplasms | Details |
| 14C-labeled poziotinib | Phase 1 Clinical | Spectrum Pharmaceuticals | Solid tumours | Details | |
| Recombinant anti-HER2 human monoclonal antibody-DM1 (Hisun Pharm/Mabworks Biotech) | HS-630; HS630 | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd, Beijing Mabworks Biotech Co Ltd | Breast Neoplasms | Details |
| Puvitinib | Phase 1 Clinical | Suzhou Teligene Ltd | Solid tumours | Details | |
| Anti-HER2 CAR T-cell therapy (Seattle Children's Hospital) | Phase 1 Clinical | Seattle Children'S Research Institute | Central Nervous System Neoplasms | Details | |
| [89Zr]-Df-Trastuzumab | Phase 1 Clinical | University Of Alabama At Birmingham | Solid tumours; Neoplasms; Breast Neoplasms | Details | |
| KSP-QRH-E3-IRDye800 | Phase 1 Clinical | University Of Michigan | Cholangiocarcinoma | Details | |
| Pertuzumab biosimilar(SL Pharma/Combio) | Phase 1 Clinical | Beijing Kangming Bio New Drug Development Co Ltd, Beijing Sl Pharmaceutical Co Ltd | Inflammatory Breast Neoplasms; Breast Neoplasms; Metastatic breast cancer | Details | |
| Recombinant Humanized Anti-Human Epidermal Growth Factor Receptor Monoclonal Antibody and the Dolastatins Derivative DUO5 Conjugated | ZV0203; ZV203; ADC2122; ADC 2122 | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd, Zhejiang Zova Biotherapeutics Inc, Concortis Biosystems Corp | Solid tumours | Details |
| 4-1BBz CD19-Her2tG (Seattle Children's Hospital) | Phase 1 Clinical | Seattle Children'S Hospital | Leukemia; Neoplasms; Lymphoma | Details | |
| HER2.taNK | NK-92/5.28.z | Phase 1 Clinical | Nantkwest Inc | Glioblastoma | Details |
| PB-357 | PB-357 | Phase 1 Clinical | Pfizer Inc | Neoplasms | Details |
| Trastuzumab biosimilar (BioIntegrator) | BI-Mab-03 | Phase 1 Clinical | Biointegrator Llc | Breast Neoplasms | Details |
| BAY-2701438 | BAY-2701438 | Phase 1 Clinical | Bayer AG | Neoplasms | Details |
| Neptinib Di-P-methylbenzenesulfonate | Phase 1 Clinical | Shenzhen Neptunus Pharmaceutical Research Institute Co Ltd | Stomach Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| BVAC-B vaccine (Cellid) | BVAC-B | Phase 1 Clinical | Cellid Company | Stomach Neoplasms | Details |
| Trastuzumab biosimilar (Hualan Biological Engineering) | Phase 1 Clinical | Hualan Genetic Engineering Co Ltd | Stomach Neoplasms; Breast Neoplasms | Details | |
| GB-251 | GB-251; NBT-828; GB221-NBT828 ADC-1 | Phase 1 Clinical | Genor Biopharma Co Ltd, Newbio Therapeutics Inc | Breast Neoplasms; Metastatic breast cancer | Details |
| HER.CAR-CMV-specific-CTLs | HER.CAR-CMV-specific-CTLs | Phase 1 Clinical | Baylor College Of Medicine | Glioblastoma | Details |
| ALT-P7 | HM2-MMAE; ALT-P7 | Phase 1 Clinical | Alteogen Inc | Stomach Neoplasms; Breast Neoplasms | Details |
| Autologous AdHER2 dendritic cell vaccine (National Cancer Institute) | Phase 1 Clinical | National Cancer Institute | Neoplasms; Breast Neoplasms; Adenocarcinoma; Neoplasm Metastasis | Details | |
| NC-18 | NC-18 | Phase 1 Clinical | Novacyte Therapeutics Company Ltd | Solid tumours | Details |
| BC-004 | BC-004 | Phase 1 Clinical | Shandong Buchang Pharmaceuticals Co Ltd | Solid tumours; Breast Neoplasms | Details |
| Trastuzumab biosimilar (United BioPharma) | UB-921 | Phase 1 Clinical | United Biopharma Inc | Breast Neoplasms | Details |
| TGFBeta-resistant-HER2-EBV-CTLs | TGFBeta-resistant-HER2-EBV-CTLs | Phase 1 Clinical | Baylor College Of Medicine | Neoplasms | Details |
| ORM-5029 | ORM-5029 | Phase 1 Clinical | Orum Therapeutics Inc | Solid tumours; Breast Neoplasms | Details |
| NeuCeptin | NeuCeptin | Phase 1 Clinical | Neuclone, Serum Institute Of India Ltd | Stomach Neoplasms; Breast Neoplasms | Details |
| XMT-2056 | XMT-2056 | Phase 1 Clinical | Mersana Therapeutics Inc | Stomach Neoplasms; Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Trastuzumab biosimilar | CMAB-809 | Phase 1 Clinical | Taizhou Maibo Taike Biotechnology Co Ltd | Breast Neoplasms | Details |
| Zanidatamab zovodotin | ZW-49 | Phase 1 Clinical | Zymeworks Inc | Neoplasms | Details |
| Sirotinib Maleate | XZP-5491 | Phase 1 Clinical | Shandong Xuanzhu Pharmaceutical Technology Co Ltd | Stomach Neoplasms; Carcinoma, Squamous Cell; Carcinoma, Non-Small-Cell Lung | Details |
| IAM-1363 | IAM-H1; ENT-H1; IAM1363; IAM-1363 | Phase 1 Clinical | Iambic Therapeutics Inc | Neoplasms | Details |
| Human HER2-targeted CAR-M cells therapy(Macera therapeutics) | Phase 1 Clinical | Nanjing Yuanmai Cell Biotechnology Co Ltd, The First People'S Hospital Of Hangzhou, Zju | Stomach Neoplasms | Details | |
| AB-201(GC Cell) | Phase 1 Clinical | GC Cell Corp | Neoplasms | Details | |
| [14C]-PLB1004 | [14C]-PLB1004 | Phase 1 Clinical | Beijing Avistone Pharmaceuticals Biotechnology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| CT-0525 | CT-0525 | Phase 1 Clinical | Carisma Therapeutics Inc | Solid tumours | Details |
| Anti-HER2-CAR-T Cells Therapy(Simcere Pharmaceutical) | Phase 1 Clinical | China Medical University, China | Solid tumours | Details | |
| IKS014 | IKS014; IKS-014 | Phase 1 Clinical | Iksuda Therapeutics Inc | Stomach Neoplasms; Esophageal Neoplasms; Digestive System Neoplasms; Breast Neoplasms; Gastrointestinal Neoplasms | Details |
| D3L-001 | D3L-001 | Phase 1 Clinical | D3 Bio (Wuxi) Co Ltd | Solid tumours; Neoplasms | Details |
| SNC-109 | SNC109; SNC-109 | Phase 1 Clinical | Shanghai Simnova Biotechnology Co Ltd | Glioblastoma | Details |
| XZP-KM501 | XZP-KM501; KM-501 | Phase 1 Clinical | Xuanzhu (Shijiazhuang) Biotechnology Co Ltd | Solid tumours; Neoplasm Metastasis | Details |
| SMP-656 | SMP-656 | Phase 1 Clinical | Xiling Lab Co Ltd | Solid tumours; Neoplasms | Details |
| ELVN-002 | ELVN-002 | Phase 1 Clinical | Enliven Therapeutics Inc | Solid tumours; Stomach Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Metastatic breast cancer; Carcinoma, Non-Small-Cell Lung | Details |
| 99mTc-MIRC213 | 99-mTc-MIRC-213 | Phase 1 Clinical | Peking Union Medical College Hospital | Breast Neoplasms | Details |
| TY4028 | TY-4028; TY4028 | Phase 1 Clinical | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details | |
| FT-825 | ONO-8250; FT-825; ONO 8250/FT 825 | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Solid tumours | Details |
| Recombinant human ErbB3 fragment vaccine (Zensun) | rhErbB3-f | Phase 1 Clinical | Zensun (Shanghai) Sci&Tech Co Ltd | Neoplasms | Details |
| SNK-02 | SNK-02; SNK02 | Phase 1 Clinical | NKGen Biotech Inc | Solid tumours; Neoplasms; Neoplasm Metastasis | Details |
| KMHH-03 | KMHH-03; KMHH03 | Phase 1 Clinical | Beijing Kangming Haihui Biological Technology Co Ltd | Breast Neoplasms | Details |
| HF-158-K1 | HF158K1; HF-158-K1; HF-158K1 | Phase 1 Clinical | Hangzhou Gaotian Biological Medicine Co Ltd | Solid tumours | Details |
| FDA022 Antibody Drug Conjugate | Phase 1 Clinical | Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co Ltd | Solid tumours | Details | |
| I-025-A | I-025-A | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Breast Neoplasms | Details |
| Pertuzumab biosimilar (biocad) | BCD-178 | Phase 1 Clinical | Biocad | Breast Neoplasms | Details |
| 99mTc- ADAPT6 | 99mTc- ADAPT6; ADAPT6; SNA-018 | Phase 1 Clinical | Uppsala University, Kth Royal Institute Of Technology | Breast Neoplasms | Details |
| 99mTc-HPArk2 | 99mTc-HPArk2; 99-mTc-HPArk-2 | Phase 1 Clinical | Peking Union Medical College Hospital | Breast Neoplasms | Details |
| 99mTc-HE3-G3 | 99mTc-HE3-G3; 99-mTc-HE-3-G-3; 99m-Tc-HE-3-G-3; 99mTc-(HE)3-G3 | Phase 1 Clinical | Tomsk National Research Medical Center Of The Russian Academy Of Sciences | Solid tumours; Breast Neoplasms | Details |
| BAY-2701439 | BAY-2701439 | Phase 1 Clinical | Bayer AG | Neoplasms | Details |
| XZP-KM257 | XZP-KM257; KM257/252; KM-257 | Phase 1 Clinical | Beijing Kangming Bio New Drug Development Co Ltd | Solid tumours | Details |
| PM-3002 | PM-3002 | Phase 1 Clinical | Biotheus Inc | Details | |
| XZP-5209 | XZP-5209 | Phase 1 Clinical | Xuanzhu (Shijiazhuang) Biotechnology Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| GQ-1005 | GQ-1005; GQ1005 | Phase 1 Clinical | GeneQuantum Healthcare (Suzhou) Co Ltd | Solid tumours; Neoplasms | Details |
| SAR-443216 | SAR-443216 | Phase 1 Clinical | Sanofi | Solid tumours; Stomach Neoplasms; Neoplasms; Breast Neoplasms; Lung Neoplasms | Details |
| NIP-142 | NIP-142 | Phase 1 Clinical | National Institutes Of Pharmaceutical Research And Development Co Ltd | Carcinoma, Non-Small-Cell Lung | Details |
| TAS-2940 | TAS-2940 | Phase 1 Clinical | Taiho Oncology Inc | Solid tumours; Glioblastoma; Breast Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Anti-HER2 CAR T-cell therapy (Baylor College of Medicine/The Hospital for Sick Children/Texas Children's Hospital/The Methodist Hospital System ) | Phase 1 Clinical | Baylor College Of Medicine, The Hospital For Sick Children, Texas Children'S Hospital, The Methodist Hosp Research Institute | Sarcoma | Details | |
| SSGJ-705 | 705; SSGJ-705 | Phase 1 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Solid tumours; Neoplasms | Details |
| HER-2 Specific CAR T Cell | Phase 1 Clinical | Pediatric Brain Tumor Consortium (Pbtc), St. Jude Children'S Research Hospital | Ependymoma | Details | |
| SPH-5030 | SPH-5030 | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Solid tumours | Details |
| 89Zr-ss-Pertuzumab | Phase 1 Clinical | Genentech Inc | Breast Neoplasms; Metastatic breast cancer | Details | |
| SSGJ-612 | SSGJ-612 | Phase 1 Clinical | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | Solid tumours | Details |
| Pertuzumab biosimilar(EirGenix) | EG-1206A; EG-1206-A | Phase 1 Clinical | Eirgenix Inc | Breast Neoplasms | Details |
| Dual-targeting HER2 and PD-L1 CAR-T Cell Therapy (Sichuan University) | Phase 1 Clinical | Sichuan University | Peritoneal Neoplasms; Pleural Effusion, Malignant | Details | |
| CT-0508 | CT-0508 | Phase 1 Clinical | Carisma Therapeutics Inc | Solid tumours | Details |
| CCT303-406 | Phase 1 Clinical | Shanghai Sinobioway Sunterra Biotech | Ovarian Neoplasms; Solid tumours; Stomach Neoplasms; Breast Neoplasms; Sarcoma | Details | |
| BAT-1006 | BAT-1006 | Phase 1 Clinical | Bio-Thera Solutions Ltd | Solid tumours | Details |
| EX-101 | EX-101; EX101 | Phase 1 Clinical | Guangzhou Excelmab Inc | Solid tumours | Details |
| DZD-1516 | DZD-1516 | Phase 1 Clinical | Dizal (Jiangsu) Pharmaceutical Co Ltd | Breast Neoplasms; Metastatic breast cancer | Details |
| MBS-301 | MBS-301 | Phase 1 Clinical | Beijing Mabworks Biotech Co Ltd | Solid tumours; Stomach Neoplasms; Breast Neoplasms | Details |
| ACE-1702 | ACE-1702 | Phase 1 Clinical | Acepodia Taiwan Subsidiary | Solid tumours; Stomach Neoplasms; Metastatic breast cancer; Neoplasm Metastasis | Details |
| Pertuzumab biosimilar (Hengrui Pharma) | SHR-1309 | Phase 1 Clinical | Jiangsu Hengrui Medicine Co Ltd | Metastatic breast cancer | Details |
| Recombinant anti-HER2 humanized monoclonal antibody (Shenzhen Main Luck Pharmaceuticals) | Phase 1 Clinical | Shenzhen Main Luck Pharmaceuticals Inc | Breast Neoplasms | Details | |
| Recombinant anti-HER2 domain II humanized monoclonal antibody (Qilu Pharmaceutical) | Phase 1 Clinical | Qilu Pharmaceutical Co Ltd | Breast Neoplasms | Details | |
| Trastuzumab biosimilar (BioXpress) | BXT-2318; BX-2318 | Clinical | Bioxpress Therapeutics Sa | Stomach Neoplasms; Metastatic breast cancer | Details |
| IBPM003TZ (Inbiopro Solutions) | IBPM-003TZ | Clinical | Inbiopro Solutions | Breast Neoplasms | Details |
| IBPM002BZ (Inbiopro Solutions) | IBPM-002BZ | Clinical | Inbiopro Solutions | Carcinoma, Renal Cell; Small Cell Lung Carcinoma; Colorectal Neoplasms | Details |
| 99mTc-DARPinG3 | 99mTc-DARPinG3; 99mTc-DARPinG-3 | Clinical | Tomsk National Research Medical Center Of The Russian Academy Of Sciences | Breast Neoplasms | Details |
| 99mTc-MIRC208 | Clinical | Beijing Cancer Hospital | Neoplasms | Details | |
| [18F]GE-226 | GE-226-[18F]; Fluorine-18-GE-226; [18F]GE-226 | Affibody Ab | Details |
This web search service is supported by Google Inc.






