 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| ID | Components | Size |
| RAS207-C01 | Pre-coated Anti-Influenza B (HA) Antibody Microplate | 1 plate |
| RAS207-C02 | Influenza B/Victoria Lineage (HA) Standard | 20 μg |
| RAS207-C03 | HRP-Anti-Influenza B (HA) Antibody | 20 μg |
| RAS207-C04 | 10×Washing Buffer | 50 mL |
| RAS207-C05 | 2×Dilution Buffer | 50 mL |
| RAS207-C06 | Substrate Solution | 12 mL |
| RAS207-C07 | Stop Solution | 7 mL |
This kit is developed for specific quantitative detection of influenza B virus HA protein in samples. This kit has been tested to specifically identify influenza B virus Victoria and Yamagata lineages HA protein.
It is for research use only.
2. Find the expiration date on the outside packaging and do not use reagents past their expiration date.
3. The opened kit should be stored per components table. The shelf life is 30 days from the date of opening.
Your experiment will include 5 simple steps:
a) Bring all reagents and samples to room temperature (20℃-25℃) before use.
b) Add your sample to the plate, take the Influenza B/Victoria Lineage (HA) as Control sample. The samples and Control sample are diluted by Dilution Buffer.
c) Add the HRP-Anti-Influenza B (HA) Antibody diluted by Dilution Buffer to the plate.
d) Wash the plate and add TMB.
e) Stop the substrate reaction by adding diluted acid. Absorbance (OD) is calculated as the absorbance at 450 nm minus the absorbance at 630 nm to remove background prior to statistical analysis. The OD Value reflects the amount of protein bound.
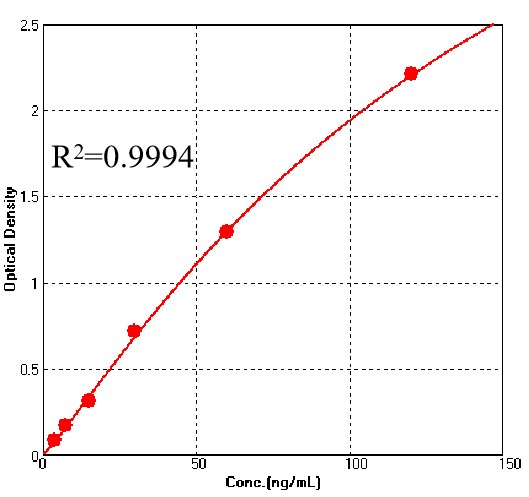
For each experiment, a standard curve needs to be set for each micro-plate, and the specific OD value may vary depending on different laboratories, testers, or equipments. The following example data is for reference only.
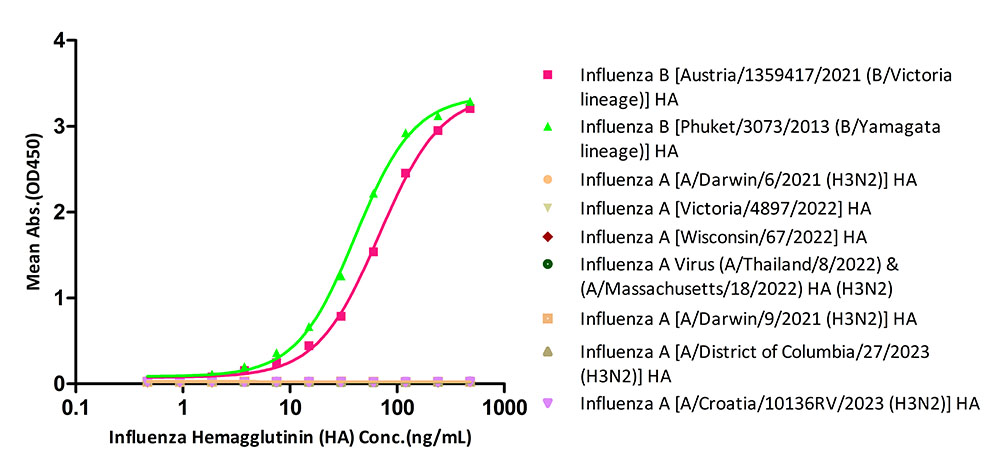
The kit has been tested to specifically identify Influenza B [Austria/1359417/2021 (B/Victoria lineage)] HA and Influenza B [Phuket/3073/2013 (B/Yamagata lineage)] HA, and do not identify Influenza A [Victoria/4897/2022 (H1N1)] HA, Influenza A [Wisconsin/67/2022(H1N1)] HA, Influenza A (A/Thailand/8/2022) & (A/Massachusetts/18/2022) HA (H3N2), Influenza A [A/Darwin/6/2021 (H3N2)] HA, Influenza A [A/Darwin/9/2021 (H3N2)] HA, Influenza A [A/District of Columbia/27/2023 (H3N2)] HA and Influenza A [A/Croatia/10136RV/2023 (H3N2)] HA.

ACROBiosystemsは、二重特異性抗体の治療薬への臨床開発を加速するために、高い生物活性を持つ均一なCD3δ/CD3εおよびCD3γ/CD3εタンパク質のシリーズを開発しました。

We offer a wide range of cell and gene therapy solutions starting from discovery to the clinic. Explore our wide range of proteins, antibodies, kits, and other assays to accelerate the development of your cell and gene therapy.

Organoid Toolbox は、即使用可能のオルガノイド、オルガノイド分化ツールキット、創薬プロジェクトの進行を加速する、さまざまなサービスを含むオルガノイドソリューションのことです。

IL-15、IL-7、IL-21など、高品質のGMP準拠サイトカイン製品を提供しており、免疫細胞治療薬の臨床研究をサポートし、医薬品規制当局の承認を加速できます。

注目されている50以上のCAR-Tターゲットがあり、CARの発現の検出のために設計された抗原タンパク質をフローサイトメトリーで検証し、CARの発現が高い特異性で検出できます。ロット間の一貫性が保証されています。また、PE / FITC標識タンパク質を利用することで、ワンステップ染色でCARの発現を高い特異性でバックグラウンドなしに検出できます。

CD20、Claudin18.2、CD133、GPRC5D、CCR5、CCR8などの安定で高活性な全長型膜貫通タンパク質は、免疫学、ELISA、SPR、BLI、細胞実験、CAR陽性率検出に利用できます。VLP、界面活性剤、ナノディスクなどのテクニカルプラットフォームによって複数回膜貫通タンパク質の医薬品創薬を促進できます。

GMP準拠サイトカイン、高品質の細胞活性化/増幅試薬、遺伝子改変試薬/酵素、CAR検出試薬などの製品を提供し、細胞、遺伝子治療用に全体的なソリューションを提供し、創薬から臨床研究までのすべての段階をサポートします。

これまでに知られている免疫チェックポイント分子をほぼ製品化しており、様々なタグの製品群を提供できます。天然高分子の構造はMALSによって、生物活性はELISA/SPR/BLI/FACSなどによって検証済みです。またBiotin/FITCなどの標識も選択でき、抗体のハイスループットスクリーニングに利用できます。

弊社ACROBiosystemsはADC医薬品の開発のサポートに取り組み、注目されている様々なターゲットのために、異なる種類とタグの製品が開発され、高い純度と親和性が特長です。免疫、抗体スクリーン、SPR、細胞活性検査などの実験に利用できます。Protocolも無償で提供いたします。

すべての分子のFc受容体タンパク質だけでなく、一般的な変異体やビオチン標識タイプも含まれております。お客様のモノクローナル抗体の開発をサポートします。

インターロイキン、成長因子、ケモカイン、TNF など、様々なサイトカインターゲットの自然なコンフォメーションを確保するために HEK293 によって発現されます。SDS-PAGE/HPLC/SEC-MALS によって高純度は確認され、高い生物活性は ELISA/SPR/BLI によって確認されています。

AneuroはACROBiosystemsが神経科学研究のために設計した製品群のブランドです。神経科学研究を推進するための治療・診断用研究タンパク質、PFF、組み換え神経因子など、高品質の重要なタンパク質を提供しております。
This web search service is supported by Google Inc.