 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
> Antibody-Drug Conjugates (ADCs): the Magic Bullets for Treatment

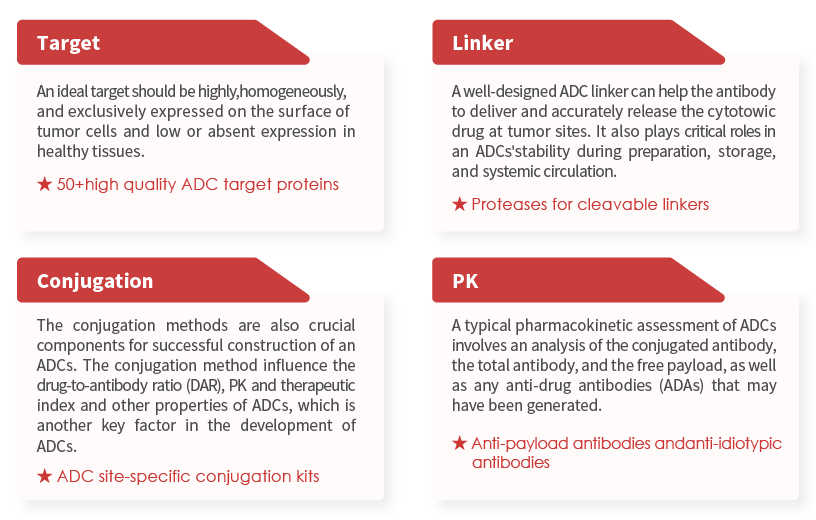
In recent years, Antibody-Drug Conjugates (ADCs) may serve as a novel therapeutic modality for many cancer patients. Indeed, three key elements define an ADC: the antibody directed against a specific tumor antigen, the cytotoxin/payload and the cleavable/uncleavable linker connecting the payload to the antibody [1]. Specific target recognition and effective toxin make ADCs veritable "magic bullet". These 'magic bullets' deliver treatment that is both highly targeted and highly potent, seeking out and attacking cancerous cells throughout the body, while avoiding harm to healthy cells. Reasonable selection of targets, antibody, linker, payload, and their rational combinations, as well as conjugation methods are crucial for an effective treatment. Among these elements, target selection, linker selection, conjugation methods and evaluating preclinical /clinical efficacy are considered critical factors for ADCs development. >>> Download the ebook: Key point of ADCs design
Features
Applications
Suitable for immunization / antibody screening / cross-reactivity/cell-based assay/QC.
Featured products
Exclusive LIV-1 proteins:
✔ Multiple species: Human, Mouse, Cynomolgus, Rat, etc.;
✔ Expression sequences were designed based on the SGN-LIV1A binding epitope.
Features
Applications
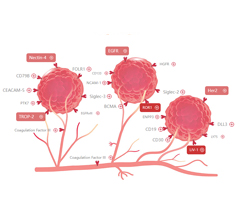
✔ Evaluate the effect of proteases cleavage of linker to ensure the efficient release of the intracellular payloads.
Features
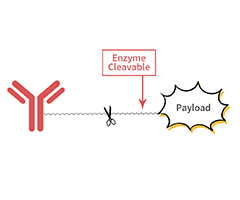
>> Site-specific conjugation through Fc-glycan with high conjugation efficiency
>> Uniform DAR value and highly homogeneous ADC products
>> Universal and efficient: No need of amino acid sequence engineering.
>> Easy to use: One-pot conjugation
>> Two options: DAR2 and DAR4 ADC kits
>> High stability of conjugates
>> Comprehensive components for easy usage, batch consistency of enzyme
Applications
✔ Conjugation of ADCs in preclinical development
Featured products
Features
>> Comprehensive products: anti-MMAE antibodies and anti-idiotypic antibodies;
>> High specificity & affinity;
>> High sensitivity & stability.
Applications
✔ Positive reference of ADCs preclinical / clinical immunogenicity;
✔ PK analysis;
✔ Anti-payload antibodies: Qualitative detection of DAR value.
Featured products
The activity of anti-MMAE monoclonal antibody (Cat. No.MME-M5252) is verified by binding to Disitamab Vedotin (RC-48) with a linear range of 0.1-2 ng/mL.
Applications
Anti-Idiotypic Antibody Development Service
Service
Applications
ADC target proteins
Proteases for peptide linker
AGLink™ ADC site-specific conjugation kits
Tools for ADCs PK analysis
| Molecule | Cat. No. | Product Description | Preorder/Order |
|---|
| Molecule | Cat. No. | Product Description | Preorder/Order |
|---|
| Molecule | Cat. No. | Product Description | Preorder/Order |
|---|
| Molecule | Cat. No. | Product Description | Neutralizing activity | Application |
|---|---|---|---|---|
| Adalimu*ab | ADB-Y19 | Anti-Adalimu*ab Antibodies (AY19) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y10 | Anti-Bevacizu*ab Antibodies (AY10) (MALS verified, recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y12 | Anti-Bevacizu*ab Antibodies (AY12) (recommended for neutralizing assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y9 | Anti-Bevacizu*ab Antibodies (AY9) (recommended for ADA assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-BY13 | Biotinylated Anti-Bevacizu*ab Antibodies (AY13) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA;Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y27 | Anti-Cetuxi*ab Antibodies (AY27) (recommended for ADA assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y31 | Anti-Cetuxi*ab Antibodies (AY31) (Non-Neutralizing) | Non-Neutralizing Antibody | ADA assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y28 | Anti-Cetuxi*ab Antibodies (AY28) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-BY31 | Biotinylated Anti-Cetuxi*ab Antibodies (AY31) (recommended for PK/PD) | Non-Neutralizing Antibody | PK bridging ELISA; Indirect ELISA |
| Rituxi*ab | RIB-Y36 | Anti-Rituxi*ab Antibodies (AY36) (recommended for ADA assay) | Neutralizing Antibody | ADA assay;Neutralizing assay; Indirect ELISA |
| Rituxi*ab | RIB-Y37 | Anti-Rituxi*ab Antibodies (AY37) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA;Neutralizing assay;Indirect ELISA |
| Trastuzu*ab | TRB-Y5b | Anti-Trastuzu*ab Antibodies (AY5b) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA |
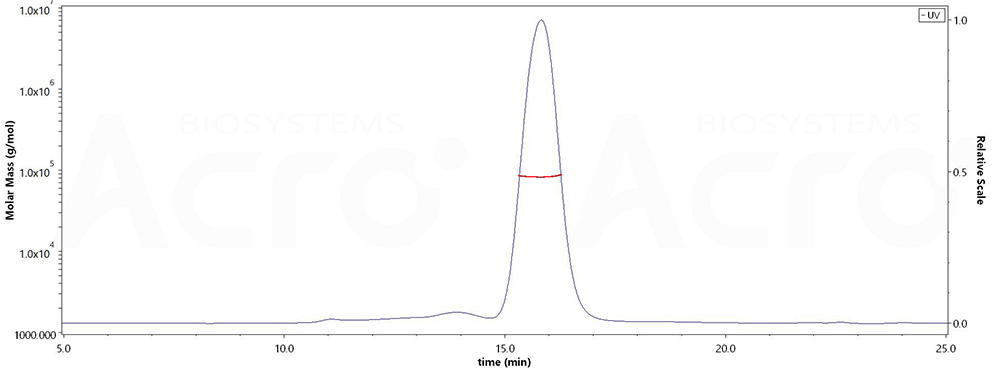
The purity of Human Her2, His Tag (Cat. No. HE2-H5225) was more than 90% and the molecular weight of this protein is around 80-95 kDa verified by SEC-MALS.
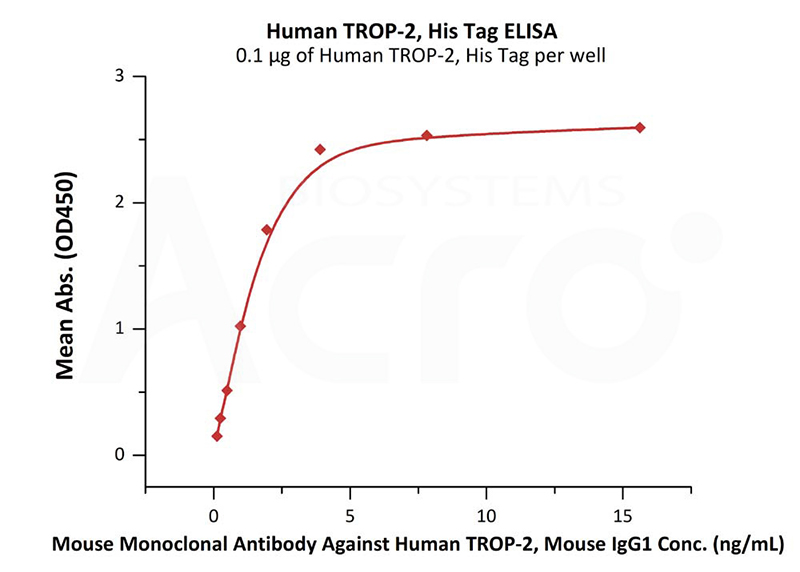
Immobilized Human TROP-2, His Tag (Cat. No. TR2-H5223) at 1 μg/mL (100 μL/well) can bind Mouse Monoclonal Antibody Against Human TROP-2, Mouse IgG1 with a linear range of 0.1-2 ng/mL (QC tested).
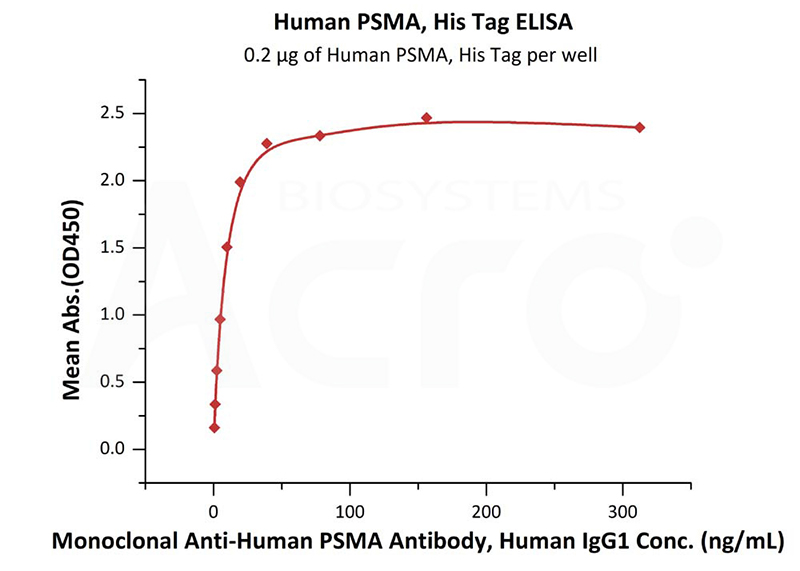
Immobilized Human PSMA, His Tag (Cat. No. PSA-H52H3) at 2 μg/mL (100 μL/well) can bind Monoclonal Anti-Human PSMA Antibody, Human IgG1 with a linear range of 0.3-39 ng/mL (Routinely tested).
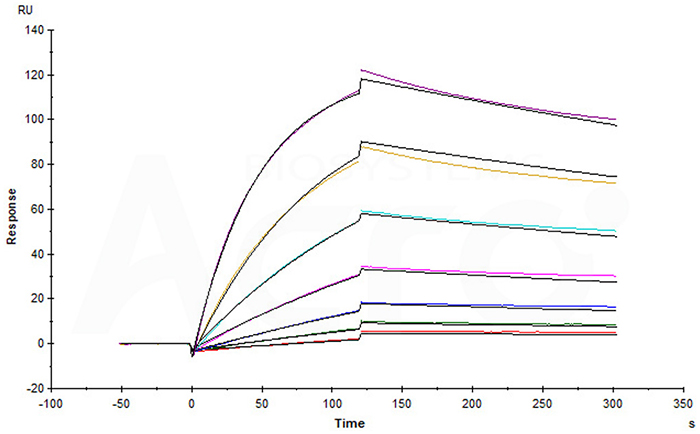
Anti-LIV-1 mAb captured on CM5 chip via anti-human IgG Fc antibody can bind Human LIV-1, His Tag (Cat. No. LV1-H5223) with an affinity constant of 5.24 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
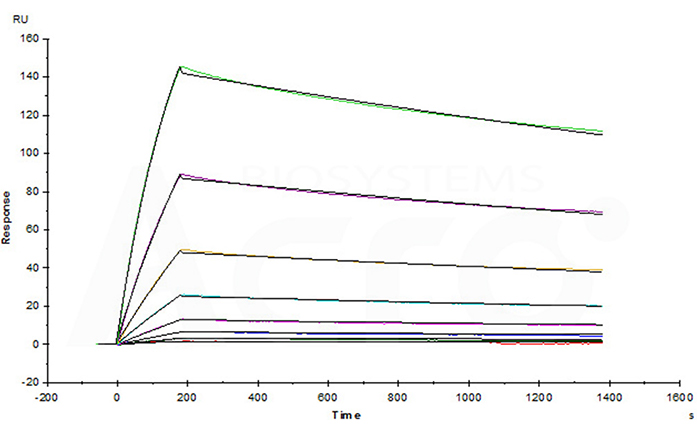
Trastuz*mab captured on CM5 chip via anti-human IgG Fc antibodies surface, can bind Human Her2, His Tag (Cat. No. HE2-H5225) with an affinity constant of 1.07 nM as determined in a SPR assay.
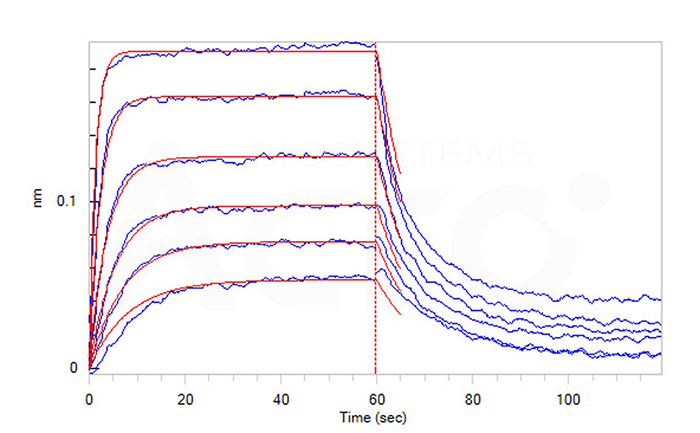
Loaded Mouse Nectin-4, His Tag (Cat. No. NE4-M52H3) on HIS1K Biosensor, can bind Human Nectin-1, Fc Tag (Cat. No. PV1-H5253) with an affinity constant of 0.12 μM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
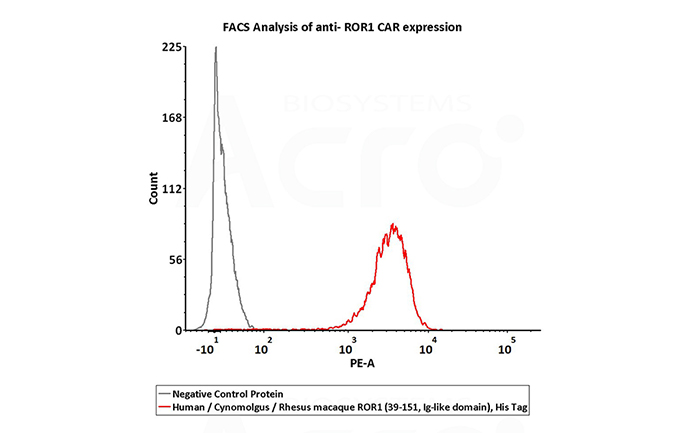
2e5 of anti-ROR1 CAR-293 cells were stained with 100 μL of 10 μg/mL of Human / Cynomolgus / Rhesus macaque ROR1 (39-151, Ig-like domain), His Tag (Cat. No. RO1-H5221) and negative control protein respectively, washed and then followed by PE-anti-His Tag and analyzed with FACS (Routinely tested).
 More anti-payload antibodies (Anti-Dxd antibody/Anti-SN-38 antibody/Anti-SN-38 antibody) are coming soon!
More anti-payload antibodies (Anti-Dxd antibody/Anti-SN-38 antibody/Anti-SN-38 antibody) are coming soon!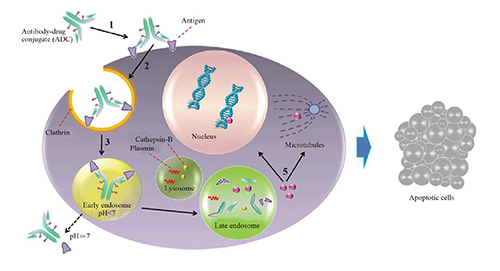
| Products | Cat. No.CTB-H5222 Human Cathepsin B / CTSB Protein, His Tag (active enzyme) | Cat. No.MM9-H5221 Human MMP-9 Protein, His Tag (active enzyme) | ||
| Hydrolyzed substrate | Fluorogenic peptide substrate Z-LR-AMC | Fluorogenic peptide substrate Mca-PLGL-Dpa-AR-NH2 | ||
| Enzymatic activity (pmol/min/μg) |
> 2500 | > 2500 | ||
 AGlink ADC Conjugation Kit (MMAE, DAR4)
AGlink ADC Conjugation Kit (MMAE, DAR4)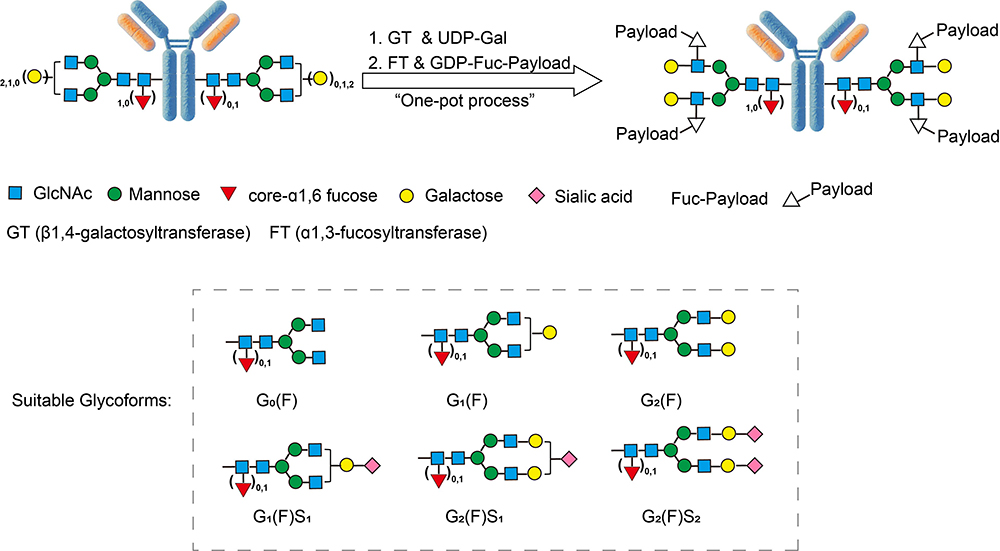
 AGlink ADC Conjugation Kit (MMAE, DAR2)
AGlink ADC Conjugation Kit (MMAE, DAR2)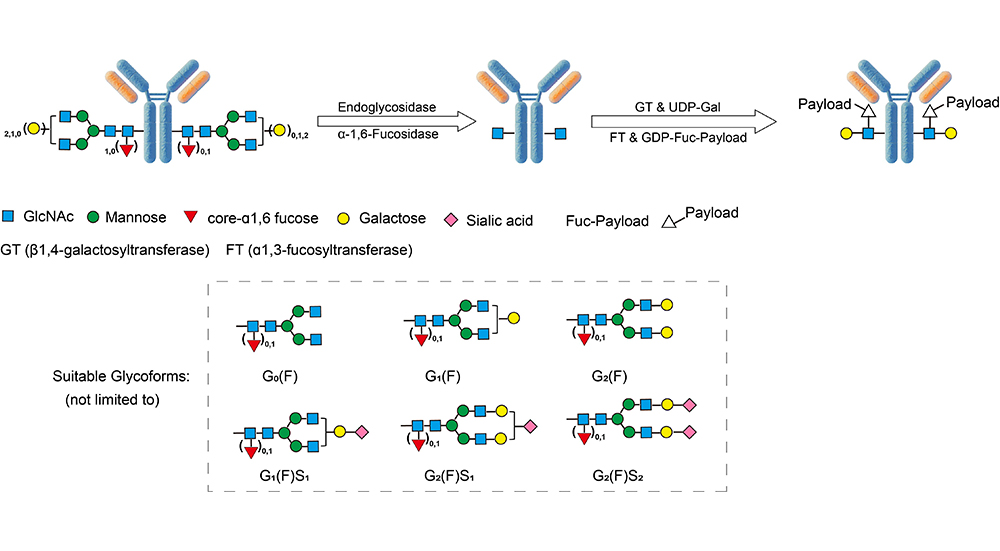
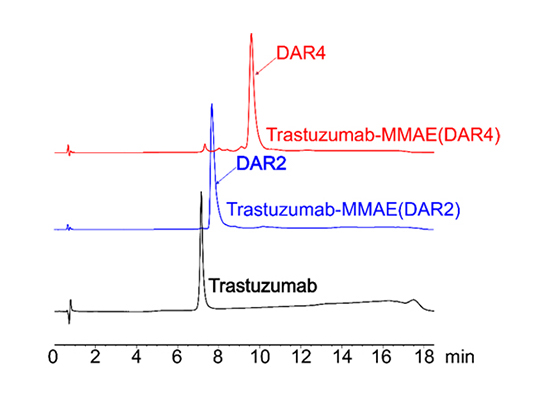
HIC-HPLC analysis of MMAE ADCs (DAR 4 & DAR 2)
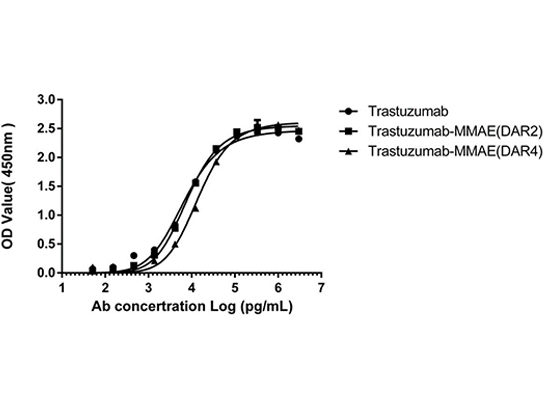
The analysis of the antigen-binding capacity of MMAE ADCs (DAR 4 & DAR 2), the results showing that binding to the HER2 antigen unaffected by the AGLink conjugation
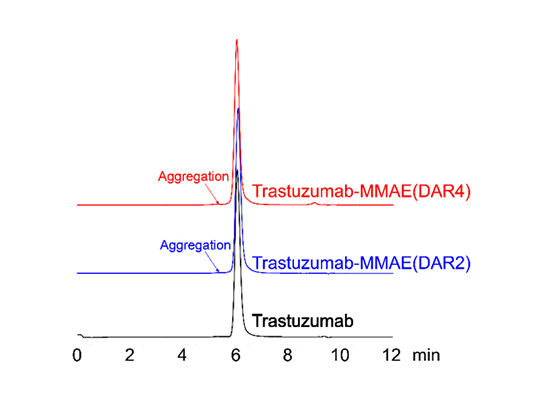
Less than 5% of antibody aggregation by SEC-HPLC
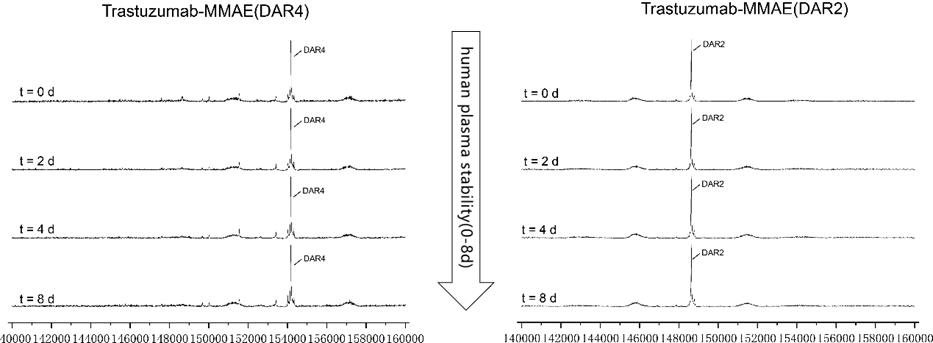
MMAE ADCs (DAR 4 & DAR 2) are stable in human plasma in vitro
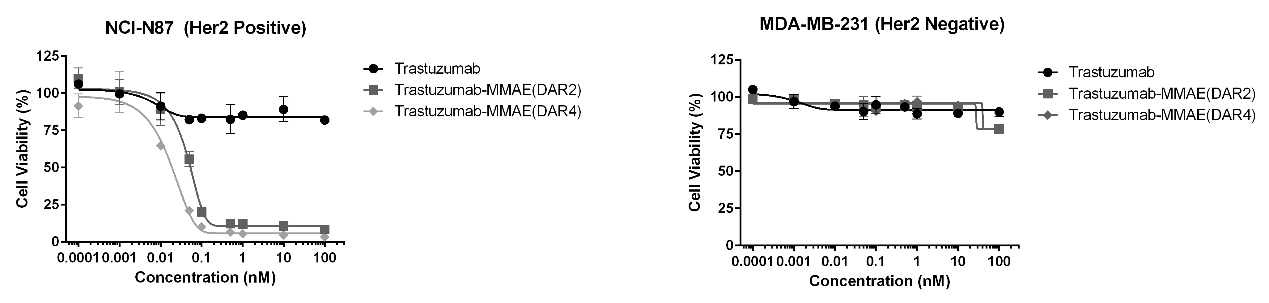
Validated in-vitro cell-killing activity of MMAE ADCs (DAR 4 & DAR 2)
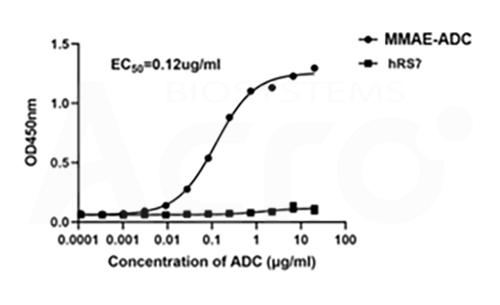
The EC50 value of anti-MMAE antibody to MMAE-conjugated hRS7 (MMAE-ADC) was 0.12 μg/ml. The unconjugated hRS7 antibody was used as negative control
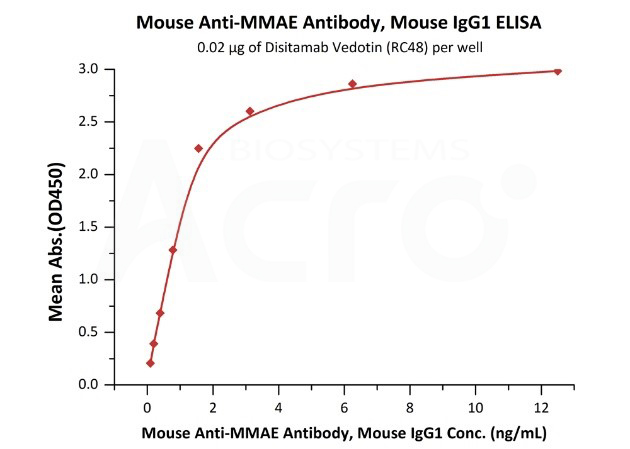
Immobilized Disitamab Vedotin (RC48) at 0.2 μg/mL (100 μL/well) can bind Mouse Anti-MMAE Antibody, Mouse IgG1 (Cat. No. MME-M5252) with a linear range of 0.1-2 ng/mL (QC tested).
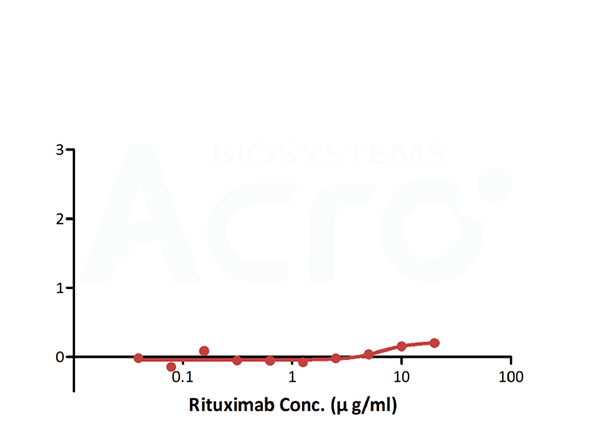
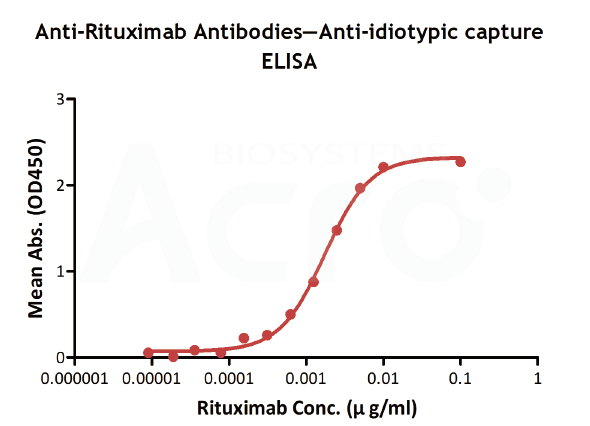
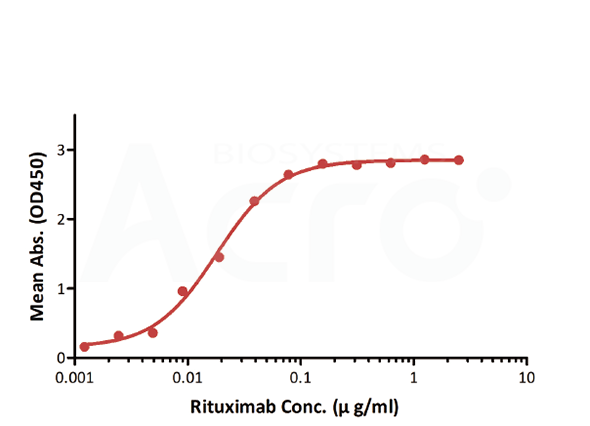
| Method | Coated | Sample | Linear range (µg/mL) |
Sensitivity (µg/mL) |
Advantage |
|---|---|---|---|---|---|
| Antigen capture ELISA | CD20 | Goat anti-human IgG | — | — | Simple and universal method |
| Anti-idiotypic capture ELISA | Anti-Ritux*mab Antibodies | Goat anti-human IgG | 0.156-10 | 0.156 | It is a simple method for extracting CD20 |
| Bridging ELISA by anti-idiotypic antibodies | Anti-Ritux*mab Antibodies | Biotinylated Anti-Ritux*mab Antibodies | 0.012-0.78 | 0.012 | CD20 can be obtained with good sensitivity and low background noise |
 SPR/BLI analytical service
SPR/BLI analytical service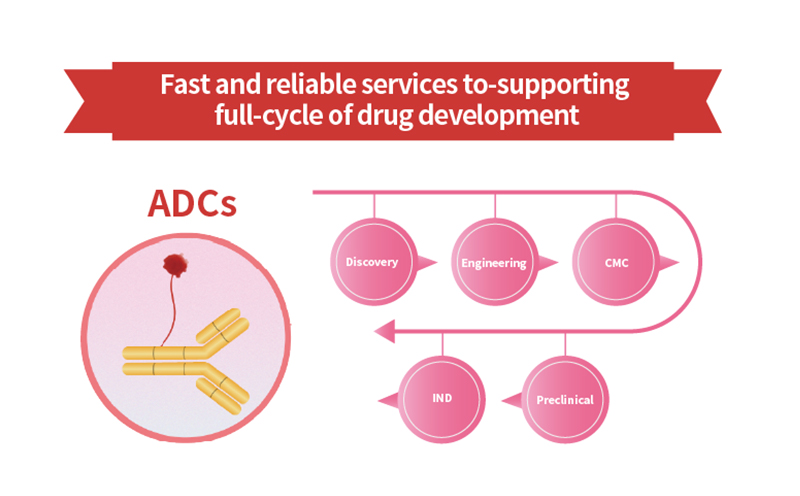
 Anti-idiotypic antibody development service
Anti-idiotypic antibody development service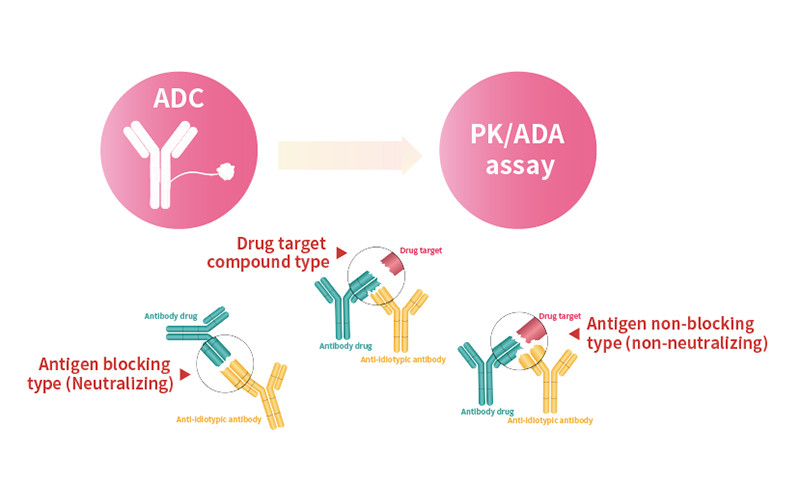
This web search service is supported by Google Inc.